Students can refer to the following Sample Paper ICSE Class 10 Chemistry Set J with Answers provided below based on the latest syllabus and examination guidelines issued for ICSE Chemistry. All specimen papers have been prepared covering all chapters given in ICSE Chemistry book for Class 10. You should also refer to ICSE Class 10 Chemistry Solutions.
Sample Paper ICSE Class 10 Chemistry Set J with Answers
Subject: Chemistry
Answers to this Paper must be written on the paper provided separately.
You will not be allowed to write during the first 15 minutes.
This time is to be spent in reading the question paper.
The time given at the head of this Paper is the time allowed for writing the answers.
Section 1 is compulsory. Attempt any four questions from Section II.
The intended marks for questions or parts of questions are given in brackets [ ].
This paper consists of 7 questions on 9 pages.
Sample Paper ICSE Class 10 Chemistry Set J
Section I (40 Marks)
Attempt all questions from this section.
Question 1
(a) Choose the correct option for the following: [10]
i) Three electrolysis cells are set up. Each cell has inert electrodes.
The electrolytes are listed below.
cell 1 aqueous sodium chloride
cell 2 dilute sulphuric acid
cell 3 molten lead (II) bromide
In which of these cells is a gas formed at both electrodes?
A) 1 and 2 B) 1 and 3 C) 2 only D) 3 only
ii) Acids are compounds which donate protons (hydrogen ions).
NH3(aq) + H2O(l) → NH4+ (aq) + OH– (aq)
Which compound in this equation is behaving as an acid?
A) ammonia
B) ammonium hydroxide
C) none of them
D) water
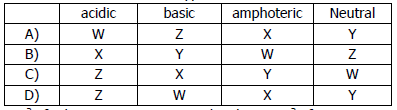
iii) The reactions of four different oxides W, X, Y and Z are shown.
W reacts with hydrochloric acid but not sodium hydroxide.
X reacts with both hydrochloric acid and sodium hydroxide.
Y does not react with either hydrochloric acid or sodium hydroxide.
Z reacts with sodium hydroxide but not hydrochloric acid.
iv) 20cm3 of ethyne, C2H2, are reacted with 500cm3 of oxygen.
The equation for the reaction is
2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(l)
What is the total volume of gas remaining at the end of the reaction? (all volume are measured at room temperature and pressure)
A) 400cm3
B) 450cm3
C) 490cm3
D) 520cm3
v) A solution contains barium ions and silver ions and one type of anion. What could the anion be?
A) chloride only
B) nitrate only
C) sulphate only
D) chloride or nitrate or sulphate
vi) Which statement explains why aluminium is used to manufacture aircraft?
A) It has a low density
B) It is a good conductor of electricity
C) It is a good conductor of heat
D) it is ductile
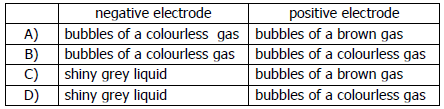
vii) What is observed at each electrode when molten lead (II) bromide is electrolysed using platinum electrodes?
viii) Which methods are suitable for preparing both zinc sulphate and cooper(II) sulphate?
1. reacting the metal oxide with warm dilute aqueous sulfuric acid
2. reading the metal with dilute aqueous sulfuric acid
3. reacting the metal carbonate with dilute aqueous sulfuric acid
A) 1, 2 and 3 B) 1 and 2 only C) 1 and 3 only D) 2 and 3 only
ix) The first members of a homologous series are shown.
Why do these molecules represent a homologous series?
A) because they contain fluorine and carbon atoms
B) because they have saturated bonds
C) because they have the same functional group
D) because they react differently from each other

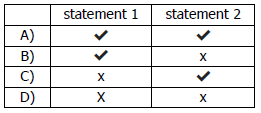
x) Which statements about Group I and Group VII elements are correct?
1 In Group I, lithium is more reactive than potassium.
2 In Group II, chlorine is more reactive than fluorine.
(b) State what do you observe when: [5]
i) Ammonium hydroxide solution is added to copper nitrate solution first little and then in excess.
ii) Concentrated sulphuric acid is added to copper sulphatecrystals.
iii) The gas obtained when excess chlorine reacts with ammonia is passed over silver nitrate solution.
iv) The acid formed when sulphur reacts with concentrated nitric acid is made to react with iron sulphide.
v) Methyl orange solution is added to sodium hydroxide solution.
(c) 1) Ethane reacts with chlorine in the presence of ultraviolet light to give a number of different compounds.
A 1.00g sample of one of these compounds contains 0.040g of Hydrogen, 0.242g of carbon and 0.718g of chlorine.
i) Calculate the empirical formula of this compound.
ii) If the relative molecular mass of the compound is 99.
Deduce the molecular formula of the compound.
2) Draw the isomers of pentane. [2]
(d) Write balanced chemical equations with suitable conditions wherever necessary for the following reactions.[5]
i) Ethanol reacts with sodium.
ii) Sulphur reacts with concentrated sulphuric acid.
iii) Calcium nitride is treated with warm water
iv) Conversion of oleum to sulphuric acid.
v) Calcium bisulphite reacts with dilute hydrochloric acid.
(e) 1) Give reason: [3]
i) Hydrogen chloride gas fumes in moist air.
ii) Electron affinity increases across a period.
iii) Non polar covalent compounds do not conduct electricity.
2) Identify the anion present in the following: [2]
i) To solution ‘X’ few drops of freshly prepared ferrous sulphate solution is added followed by concentrated sulphuric acid from the sides of the test tube. A brown ring is formed at the junction for the two liquids.
iii) Compound ‘Y’ reacts with HCl to give a gas which turns moist lead acetate paper silvery black.
(f) Answer the following questions: [5]
i) Name the alloy of lead used in electrical fuse.
ii) State the special property of the element in above alloy.
iii) Among the following elements Li, Na, K, Rb, Cs which element will form ions readily? Why?
iv) Identify the cation in a solution which gives white precipitate with ammonium hydroxide but the precipitate is insoluble in excess of ammonium hydroxide.
(g) 1) Draw the structure of the following organic compounds: [3]
i) 2-methyl pentanal
ii) 2,2,3,3 tetra bromo butanol
iii) 4-chloro-4-methyl pent-2-yne
2) Define the following: [2]
i) Mole
ii) Co-ordinate bond
Sample Paper ICSE Class 10 Chemistry Set J
Section B (40 marks)
Attempt any four out of six questions.
Question 2
(a) Electroplating steel objects with silver involves a three-step process.[6]
Step 1 A coating of copper is applied to the object.
Step 2 A coating of nickel is applied to the object.
Step 3 The coating of silver is applied to the object.
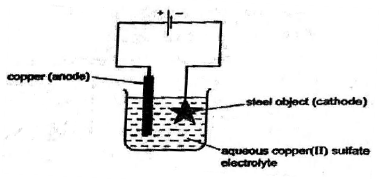
1) A diagram of the apparatus used for step 1 is shown.
i) Write the reaction taking place at the cathode.
ii) State the type of reaction taking place at the anode. Justify.
iii) Explain why the concentration of copper ions in the electrolyte remains constant throughout step 1.
2) Give two changes which would be needed in order to coat nickel on to the object in step 2.
3) What are the components of stainless steel?
(b) Substance H is an unknown salt. The figure shows a description Written by students of how they attempted to identify the salt.
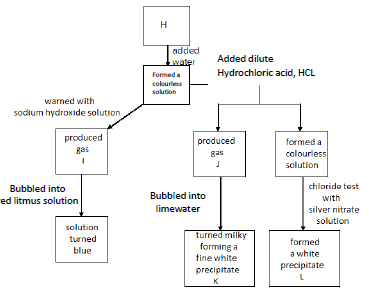
i) Name the gases I and J and the white precipitates K and L.
ii) Write a chemical equation to represent the reaction between H and sodium hydroxide.
iii) What would you observe when excess of ammonium hydroxide is added to precipitate L?
Question 3
(a) How will you prepare the following salts in the laboratory? [6]
Name the method and write chemical equation for each of them.
i) Ferric chloride
ii) Potassium nitrate
iii) Magnesium carbonate
iv) Copper sulphate
(b) Ethene and chlorine reacts in the presence of inert solvent to form dichloroethane.[4]
i) What type of reaction takes place between ethene and chlorine?
ii) Draw the structure of dichloroethane.
iii) Give balanced chemical equation to convert dichloro ethane to ethyne.
iv) Give one use of ethyne apart from fuel.
Question 4
(a) Hydrogen chloride gas dissolves in water to form solution ‘X’. [6]
i) Name the stable positive ion present in solution ‘X’.
ii) Draw the structure of this positive ion.
iii) Write a reaction to convert the negative ion to a molecule.
iv) State the suitable method of dissolving HCI gas in water.
v) Write balanced equation of a saturated solution of ‘X’ with: manganese (IV) oxide.
vi) What do you observe in the above reaction?
(b) Distinguish between oxyacids and hydracids. [1]
(c) Element ‘X with valency 3 combines with a non metal with valency 2.[3]
i) What type of bond is formed between the two elements?
ii) Will the compound formed have a high or low boiling point? Why?
iii) Write down the reaction taking place between X and Y.
Question 5
(a) Potassium chlorate (KClO3) on heating in the presence of a catalyst gives potassium chloride and oxygen.[2]
i) Calculate the mass of potassium chlorate required to produce 6.72 litres of oxygen at STP. (K = 39, Cl=35.5, O = 16)
ii) Calculate the number of molecules of oxygen present in the above volume.
(b) Gas ‘X’ occupies a volume of 100 cm3 and STP and weighs 0.5g Find its relative molecular mass.[1]
(c) 120 cc of oxygen was added to 48 cc of carbon monoxide and the mixture ignited. Calculate the volume of oxygen used up and the volume of carbon dioxide formed.
(d) Distinguish between: [3]
i) Sodium sulphate solution and Sodium sulphite solution
ii) Zinc nitrate and Lead nitrate solution.
iii) Sodium chloride and Potassium chloride
(e) Name the method of converting carbonate ores to its oxide. [1]
(f) Define Flux. [1]
Question 6
(a) The following questions are based on the preparation of ammonia gas in the laboratory:[3]
i) Explain why ammonium nitrate is not used in the preparation of ammonia.
ii) Name the compound normally used as a drying agent during the process. Justify your answer with a suitable reason.
iii) How is ammonia gas collected? Give a suitable reason for your answer.
(b) Name the acid prepared by the catalytic oxidation of ammonia. [1]
(c) i) Write the chemical equation for the laboratory preparation of nitric acid from Bengal Salt Petre.[3]
ii) Why should rubber stopper or cork not be used to stopper the mouth of the apparatus in the laboratory preparation of nitric acid?
iii) How does dilute nitric acid behave differently from typical dilute acids on reaction with metals.
(d) Fill in the blanks: [3]
i) Copper reacts with cold dilute nitric acid to produce ______ gas.
ii) The basicity of acetic acid is _________________.
iii) The IUPAC name of formaldehyde is _________________.
Question 7
(a) An element ‘X’ has atomic no. 20 and element ‘Y’ has atomic no.8.[5]
i) State the period and the group where element ‘Y’ is placed in the Periodic Table.
ii) Element ‘X’ or ‘Y’ will have more I.P. Justify.
iii) Draw the electron dot diagram of the formation of compound formed when element ‘X’ combines with ‘Y’.
iv) Will the element above ‘X’ be more metallic or less metallic? Give reason.
v) What will happen if the compound formed between ‘X’ and ‘Y’ is kept in a jar containing HCI gas?
(b) Name the main ore of zinc. How is it concentrated? [2]
(c) Why is Bauxite concentrated with caustic alkali? [1]
(d) A mixture of electrolyte is used in Hall Heroult’s process for the Electrolytic reduction of the ore to its metal. Why? [1]
(e) Write the reaction taking place at the anode in Hall Heroult’s process. [1]