Students should refer to ICSE Class 10 Chemistry Question Paper solved Set D given below which will help them to prepare for the upcoming ICSE Chemistry exams. Students should read ICSE Chemistry Class 10 Books to make sure they are completely prepared and should also refer to ICSE Class 10 Chemistry Solutions to understand all questions and their answers.
ICSE Class 10 Chemistry Question Paper solved Set D
PAPER 2 (CHEMISTRY)
(Two hours)
Answers to this Paper must be written on the paper provided separately.
You will not be allowed to write during the first 15 minutes.
This time is to be spent in reading the Question Paper.
The time given at the head of this paper is the time allowed for writing the answers.
Section I is compulsory. Attempt any four questions from Section II.
The intended marks for questions or parts of questions are given in brackets [ ].
ICSE Class 10 Chemistry Question Paper solved Set D
SECTION – I (40 MARKS)
(Attempt all questions from this Section)
Question 1. (a) Choose the correct answer from the options given below:
(i) Ionisation Potential increases over a period from left to right because the:
(A) Atomic radius increases and nuclear charge increases
(B) Atomic radius decreases and nuclear charge decreases
(C) Atomic radius increases and nuclear charge decreases
(D) Atomic radius decreases and nuclear charge increases.
(ii) A compound X consists of only molecules. Hence X will have:
(A) A crystalline hard structure
(B) A low melting point and low boiling point
(C) An ionic bond
(D) A strong force of attraction between its molecules.
(iii) When fused lead bromide is electrolysed we observe:
(A) a silver grey deposit at anode and a reddish brown deposit at cathode
(B) a silver grey deposit at cathode and a reddish brown deposit at anode
(C) a silver grey deposit at cathode and reddish brown fumes at anode
(D) silver grey fumes at anode and reddish brown fumes at cathode.
(iv) The main ore used for the extraction of iron is:
(A) Haematite
(B) Calamine
(C) Bauxite
(D) Cryolite
(v) Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as:
(A) smelting
(B) ore dressing
(C) calcination
(D) bessemerisation
(vi) If an element A belongs to Period 3 and Group II then it will have,
(A) 3 shells and 2 valence electrons
(B) 2 shells and 3 valence electrons
(C) 3 shells and 3 valence electrons
(D) 2 shells and 2 valence electrons
(vii) The molecule containing a triple co-valent bond is:
(A) ammonia
(B) methane
(C) water
(D) nitrogen
(viii)The electrolyte used for electroplating an article with silver is:
(A) silver nitrate solution
(B) silver cyanide solution
(C) sodium argentocyanide solution
(D) nickel sulphate solution
(ix) Aluminium powder is used in thermite welding because,
(A) it is a strong reducing agent
(B) it is a strong oxidising agent
(C) it is corrosion resistant
(D) it is a good conductor of heat.
(x) The I.U.P.A.C. name of acetylene is,
(A) propane
(B) propyne
(C) ethene
(D) ethyne.
(b) Fill in the blanks from the choices given within brackets:
(i) The basicity of Acetic Acid is (3, 1, 4)
(ii) The compound formed when ethanol reacts with sodium is (sodium ethanoate, sodium ethoxide, sodium propanoate)
(iii) Quicklime is not used to dry HCl gas because (CaO is alkaline, CaO is acidic, CaO is neutral)
(iv) Ammonia gas is collected by (an upward displacement of air, a downward displacement of water, a downward displacement of air)
(v) Cold, dilute nitric acid reacts with copper to form (Hydrogen, nitrogen dioxide, nitric oxide). [5]
(c) Give one word or phrase for the following:
(i) The ratio of the mass of a certain volume of gas to the mass of an equal volume of hydrogen under the same conditions of temperature and pressure.
(ii) Formation of ions from molecules.
(iii) Electrolytic deposition of a superior metal on a baser metal.
(iv) Hydrocarbons containing
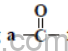
functional group.
(v) The amount of energy released when an atom in the gaseous state accepts an electron to form an anion. [5]
(d) Match the options A to E with the statements (i) to (v):
A alkynes (i) No. of molecules in 22.4 dm3 of carbon dioxide at s.t.p.

(e) Write balanced equations for the following:
(i) Action of heat on a mixture of copper and concentrated nitric acid.
(ii) Action of warm water on magnesium nitride.
(iii) Action of concentrated sulphuric acid on carbon.
(iv) Action of dilute hydrochloric acid on sodium sulphide.
(v) Preparation of ethane from sodium propionate. [5]
(f) Distinguish between the following pairs of compounds using the test given within brackets:
(i) Iron (II) sulphate and iron (III) sulphate (using ammonium hydroxide)
(ii) A lead salt and a zinc salt (using excess ammonium hydroxide)
(iii) Sodium nitrate and sodium sulphite (using dilute sulphuric acid)
(iv) Dilute sulphuric acid and dilute hydrochloric acid (using barium chloride solution)
(v) Ethane and ethene (using alkaline potassium permanganate solution) [5]
(g) (i) Oxygen oxidises ethyne to carbon dioxide and water as shown by the equation:
2C2H2 + 5O2 + 4CO2 + 2H2O
What volume of ethyne gas at s.t.p. is required to produce 8.4 dm3 of carbon
dioxide at s.t.p.? [H = 1, C = 12, O = 16]
(ii) A compound made up of two elements X and Y has an empirical formula X2Y. If the atomic weight of X is 10 and that of Y is 5 and the compound has a vapour density 25, find its molecular formula.
Answer.
(a) (i) (D) Atomic radius decreases and nuclear charge increases.
(ii) (B) A low melting point and low boiling point
(iii) (C) a silver grey deposit at cathode and reddish brown fumes at anode
(iv) (A) Haematite (v) (C) calcination
(vi) (A) 3 shells and 2 valence electrons (vii) (D) nitrogen
(viii)(C) sodium argentocyanide solution (ix) (A) it is a strong reducing agent
(x) (D) ethyne
(b) (i) The basicity of Acetic Acid is 1.
(ii) The compound formed when ethanol reacts with sodium is sodium ethoxide.
(iii) Quicklime is not used to dry HCl gas because CaO is alkaline.
(iv) Ammonia gas is collected by a downward displacement of air.
(v) Cold, dilute nitric acid reacts with copper to form nitric oxide.
(c) (i) The ratio of the mass of a certain volume of gas to the mass of an equal volume of hydrogen
under the same conditions of temperature and pressure ———→ Relative vapour density.
(ii) Formation of ions from molecules ———→ Ionisation.
(iii) Electrolytic deposition of a superior metal on a baser metal ————→ Electroplating

(v) The amount of energy released when an atom in the gaseous state accepts an electron to
form an anion ———→ Electron Affinity.

(e) (i) Action of heat on a mixture of copper and concentrated nitric acid.
Cu + 4HNO3 —→ Cu(NO3)2 + 2H2O + 2NO2
(ii) Action of warm water on magnesium nitride.
Mg3N2 + 6H2O —→ 3Mg(OH)2 + 2NH3
(iii) Action of concentrated sulphuric acid on carbon.
C + 2H2SO4 —→ CO2 + 2H2O + 2SO2
(iv) Action of dilute hydrochloric acid on sodium sulphide.
Na2S + 2HCl —→ 2NaCl + H2S
(v) Preparation of ethane from sodium propionate.
C2H5 COONa + NaO H —→ C2H6 + Na2CO3
(f) (i) Iron (II) sulphate and iron (III) sulphate (using NH4OH)
Iron (II) sulphate (Fe2+ ion)

With excess of NH4OH ppt. dissolves
Zn(OH)2 + (NH4)2SO4 + 2NH4OH → [Zn(NH3)4] SO4 + 4H2O

ICSE Class 10 Chemistry Question Paper solved Set D
SECTION – II (40 MARKS)
(Attempt any four questions from this Section)
Question 2.
(a) State your observation in each of the following cases:
(i) When dilute hydrochloric acid is added to sodium carbonate crystals.
(ii) When excess sodium hydroxide is added to calcium nitrate solution.
(iii) At the cathode when acidified aqueous copper sulphate solution is electrolyzed with copper electrodes.
(iv) When calcium hydroxide is heated with ammonium chloride crystals.
(v) When moist starch iodide paper is introduced into chlorine gas. [5]
(b) Study the figure given below and answer the questions that follow:

(i) Identify the gas Y.
(ii) What property of gas Y does this experiment demonstrate?
(iii) Name another gas which has the same property and can be demonstrated through this experiment. [3]
(c) (i) Name the other ion formed when ammonia dissolves in water.
(ii) Give one test that can be used to detect the presence of the ion produced. [2]
Answer.
(a) (i) Brisk effervescence of a gas which turns lime water milky.
(ii) A chalky white ppt insoluble in excess sodium hydroxide.
(iii) Reddish brown deposit.
(iv) Gas with a pungent odour evolved.
(v) The paper turns blue black.
(b) (i) Identify the gas Y — It is HCl (Hydrogen chloride)
(ii) Property of Y — Highly solubility in water
(iii) The another gas has same property — NH3 (Ammonia)
(c) (i) When Ammonia dissolves in water.
NH3 + H2O → NH4OH
NH4OH → NH4
- + OH–
ions formed are ammonium and hydroxyl.
(ii) Add copper sulphate solution – pale blue ppt formed or ferrous sulphate solution – dirty
green ppt formed
Question 3.
(a) State the conditions required for the following reactions to take place:
(i) Catalytic hydrogenation of ethyne.
(ii) Preparation of ethyne from ethylene dibromide.
(iii) Catalytic oxidation of ammonia to nitric oxide.
(iv) Any two conditions for the conversion of sulphur dioxide to sulphur trioxide. [5]
(b) State the main components of the following alloys:
(i) Brass.
(ii) Duralumin.
(iii) Bronze. [3]
(c) Give balanced equations for the following:
(i) Laboratory preparation of nitric acid.
(ii) Preparation of ethanol from monochloroethane and aq. sodium hydroxide. [2]
Answer.


Question 4.
(a) Give the structural formula of the following:
(i) ethanol.
(ii) 1-propanal.
(iii) ethanoic acid.
(iv) 1, 2, dichloroethane. [4]
(b) Draw the structure of the stable positive ion formed when an acid dissolves in water. [2]
(c) State the inference drawn from the following observations:
(i) On carrying out the flame test with a salt P a brick red flame was obtained. What is the cation in P?
(ii) A gas Q turns moist lead acetate paper silvery black. Identify the gas Q.
(iii) pH of liquid R is 10. What kind of substance is R?
(iv) Salt S is prepared by reacting dilute sulphuric acid with copper oxide. Identify S. [4]
Answer.

(c) (i) Cation in P is Ca2+.
(ii) The gas Q is H2S.
(iii) The substance R is alkaline.
(iv) The compound or salt S is copper sulphate CuSO4
CuO + H2SO4 —→ CuSO4 + H2O
Question 5.
(a) Name the following:
(i) The property possessed by metals by which they can be beaten into sheets.
(ii) A compound added to lower the fusion temperature of electrolytic bath in the extraction of aluminium.
(iii) The ore of zinc containing its sulphide. [3]
(b) Give one equation each to show the following properties of sulphuric acid:
(i) Dehydrating property.
(ii) Acidic nature.
(iii) As a non-volatile acid. [3]
(c) Give balanced chemical equations to prepare the following salts:
(i) Lead sulphate from lead carbonate.
(ii) Sodium sulphate using dilute sulphuric acid.
(iii) Copper chloride using copper carbonate.
Answer.

Question 6.
(a) (i) State Avogadro’s Law.
(ii) A cylinder contains 68g of ammonia gas at s.t.p.
- 1. What is the volume occupied by this gas?
- 2. How many moles of ammonia are present in the cylinder?
- 3. How many molecules of ammonia are present in the cylinder?
[N-14, H-1] [4]
(b) (i) Why do covalent compounds exist as gases, liquids or soft solids?
(ii) Which electrode: anode or cathode is the oxidising electrode? Why? [3]
(c) Name the kind of particles present in:
(i) Sodium Hydroxide solution.
(ii) Carbonic acid.
(iii) Sugar solution.
Answer.
(a) (i) Avogadro’s Law states that “equal vol. of all gases under similar conditions of temperature and pressure contain the same no. of molecules.”
(ii) 1. Molecular gram atom wt. of NH3 = 17 gm
17 gm of NH3 has vol. at s.t.p. = 22.4 lt.
68 gm of NH3 has vol. at s.t.p. = 22.4 x 68 /17 = 89.6 lt.
2. No. of moles in 68 gm of NH3 = 68 / 17 = 4 moles.
3. No. of molecules of ammonia = 4 × 6.023 × 1023 = 24.092 × 1023
(b) (i) The molecules are held together with weak vander wall’s forces. As these forces are weak, so they are gases, liquids or soft solids.
(ii) Anode – It takes up the electrons from the anions.
(c) (i) Sodium hydroxide sol. → only ions
(ii) Carbonic acid → ions and molecules
(iii) Sugar solution → only molecules
Question 7.
(a) An element Z has atomic number 16. Answer the following questions on Z:
(i) State the period and group to which Z belongs.
(ii) Is Z a metal or a non-metal?
(iii) State the formula between Z and Hydrogen.
(iv) What kind of a compound is this? [4]
(b) M is a metal above hydrogen in the activity series and its oxide has the formula M2O.
This oxide when dissolved in water forms the corresponding hydroxide which is a good conductor of electricity. In the above context answer the following:
(i) What kind of combination exists between M and O?
(ii) How many electrons are there in the outermost shell of M?
(iii) Name the group of which M belongs.
(iv) State the reaction taking place at the cathode.
(v) Name the product at the anode.
Answer.
(a) An element Z has atomic number 16
(i) Period – 3 group – 6 (as E.C. 2, 8, 6)
(ii) Z is a non-metal.
(iii) Formula between Z and hydrogen is H2Z.
(iv) The kind of compound is covalent.
(b) (i) Electrovalent combination
(ii) One
(iii) Alkali metals
(iv) Reaction of cathode: M+ + e– → M
(v) Oxygen / O2