Students can refer to the following Sample Paper ICSE Class 10 Chemistry Set E with Answers provided below based on the latest syllabus and examination guidelines issued for ICSE Chemistry. All specimen papers have been prepared covering all chapters given in ICSE Chemistry book for Class 10. You should also refer to ICSE Class 10 Chemistry Solutions.
Sample Paper ICSE Class 10 Chemistry Set E with Answers
Second Term Examination, 2020
CHEMISTRY
SCIENCE Paper – 2
Answers to this Paper must be written on the paper provided separately.
You will not be allowed to write during the first 15 minutes.
This time is to be spent in reading the Question Paper.
The time given at the head of this paper is the time allowed for writing the answers.
Section I is compulsory. Attempt any four questions from Section II.
The intended marks for questions or parts of questions are given in brackets [ ].
SECTION I ( 40 Marks )
(Attempt ALL questions)
Question 1
a)Choose the correct answer from the options given below: [5]
i)An element whose electron affinity is zero:
a) Hydrogen
b) Helium
c) Fluorine
d) Nitrogen
ii)Identify the compound that has coordinate bond:
a) Potassium chloride
b) Ammonium chloride
c) Calcium chloride
d) Ammonia
iii)The salt solution which does not react with ammonium hydroxide is:
a)Zinc nitrate
b) Copper nitrate
c) Calcium nitrate
d) Lead nitrate
iv)The electrolyte used in the electroplating of an article with silver is,
a) Silver chloride
b) Sodium chloride
c) Silver nitrate
d) Sodium argentocyanide
v) Formation of chloroform from methane and chlorine is an example of,
a) Addition
b) Dehydration
c) Elimination
d) Substitution
b)Fill in the blanks using the following words : [5]
[ salt , hydronium , ammonium , ammonia , precipitate , hydroxide , hydrogen , water ]
A solution X turns blue litmus red, so it must contain (i) ……… ions. Another solution Y turns red litmus
blue and therefore it must contain (ii) ………… ions . When solutions X and Y are mixed together, the
products will be a (iii) ………. and (iv) ……….. . If a piece of zinc metal is added to the solution of X ,
(v) ………..gas will be evolved.
c)Match the following:
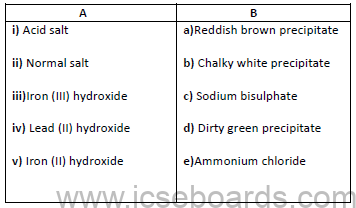
d)Give reasons: [5]
i)Electrolysis of water gives two volumes of hydrogen and one volume of oxygen.
ii)A graphite anode is preferred to other inert electrodes during electrolysis of fused lead bromide.
iii)In the electrolysis of acidified water dilute sulphuric acid is preferred to dilute nitric acid for acidification.
iv)In the electrolytic reduction of alumina the graphite anode is gradually consumed.
v)Lead bromide undergoes electrolytic dissociation in the molten state but is a non electrolyte in the solid
state.
e) Give the structural formula of the following , [5]
i)Methanol
ii)Methanal
iii)Methanoic acid
iv)But-2-ene
v) 2-Methylpropane
f)Differentiate between, [5]
i)Electrolyte and non-electrolyte
ii)Oxidation and reduction
iii) Cathode and anode
iv)cation and anion
v)Inert electrode and active electrode g)Name the following , [5]
i)Highly electronegative element of halogens.
ii) A molecule has both slight positive and slight negative charge.
iii)Metallic oxides which show bothacidic as well as basic character.
iv)Formula which gives the simplest ratio in whole numbers of atoms of different elements in one molecule of the compound.
v) Main ore of aluminium.
h)Complete and balancethe following chemical equations:[5]
i)NaHSO3 + HCl →
ii)NH4Cl + NaOH →
iii)Zn + KOH →
iv) C2H6 + Cl2 →
v)H2C=CH2 + Br2 →
SECTION II ( 40 Marks )
(Attempt any FOUR questions)
Question 2
a) A group of elements in the periodic table is given below:

i)Which element would be expected to have the highest electronegativity?
ii)Which element has the most metallic character?
iii)How many electrons are there in the outer shell of Tl?
b) The pH values of three solutions A ,B and C are given in the table
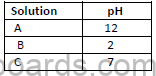
i)Which solution will change red litmus solution blue?
ii)Which solution will have no effect on litmus solution?
iii)Which solution will liberate carbon dioxide with sodium carbonate?
c)Give reasons, [4]
i)Metallic property increases down the group.
ii)Atomic size decreases across a period.
iii) Electron affinity of F is less than Cl.
iv) Ionisation potential decreases down the group.
Question3
a) When ammonium hydroxide is added to an aqueous solution of X a gelatinous white precipitate [3]
is formed which dissolves in excess to form a clear solution. Then,
i)Identify the cation present in X.
ii)Write the balanced chemical equation for the above reactions.
b)How many lone pair/s present in the following compound/ions, [3]
i) Hydronium ion
ii) Water
iii) Hydroxyl ion
c)Find the empirical formulaand the molecular formulaof an organic compound from the data [4]
givenbelow:
C = 75.92%, H = 6.32% and N = 17.76% The vapour density of the compound is 39.5.
[C = 12, H = 1, N = 14 ]
Question 4
a)0.5 g of an organic compound contains 0.062 g of hydrogenand 0.25 g of oxygen.Determine the [3]
molecular formula of the organic compound if its molecular mass is 64.
b)Give the chemical formula of: [3]
(i) Alumina
(ii) Cryolite
(iii) Fluorspar
c) Draw the electron dot structure for the formation,
i)Sodium chloride [2]
ii) Methane [2]
Question 5
a)State the observations at the anode and at the cathode during the electrolysis of, [4]
i)Aqueous copper sulphate solution using copper electrodes.
ii)Fused lead bromide using graphite electrodes.
b) Write the chemical equations involved in Bayer’s process. [3]
c)Calculate the percentage composition of various elements in CH3CH2OH. [3]
[C=12,O=16,H=1]
Question 6
a)Write the I.U.P.A.C. name of the following,

b)i)What are homologous series?Give examples. [3]
ii)Draw the chain isomers of butane.
c)Write the balanced chemical equations for the following conversions , [3]
i) Ethene to ethane
ii) Ethyne to 1,2-Dichloroethene
iii) Dichloromethane to chloroform.