Students can refer to the following Sample Paper ICSE Class 10 Chemistry Set H with Answers provided below based on the latest syllabus and examination guidelines issued for ICSE Chemistry. All specimen papers have been prepared covering all chapters given in ICSE Chemistry book for Class 10. You should also refer to ICSE Class 10 Chemistry Solutions.
Sample Paper ICSE Class 10 Chemistry Set H with Answers
Grade: X
Subject : Chemistry
• You will not be allowed to write during the first 15 minutes.
• This time is to be spent in reading the question paper.
• The time given at the head of this paper is the time allowed for writing the answers.
• This paper consists of 7 printed pages.
• Attempt all questions from Section I and any four questions from Section II.
• The intended marks for questions or parts of questions are given in brackets [ ].
Sample Paper ICSE Class 10 Chemistry Set H
SECTION I (40 Marks)
Attempt all questions from this section
Question I
(a) Select an appropriate answer from the given options and write the alphabet corresponding to the correct answer: [5]
(i) A salt which in solution gives a bluish white precipitate with NaOH and white precipitate with BaCl2 solution is:
(a) CuSO4
(b) FeSO4
(c) Fe2(SO4)3
(d) CuCl2
(ii) The resulting ester, when ethyl alcohol and acetic acid are mixed together is:
(a) CH3COOCH3
(b) C2H5COOC2H5
(c) CH3COOC2H5
(d) C2H5COOCH3
(iii) Which of the following reactions gives copper as a product?
(a) Passing dry ammonia over heated copper oxide.
(b) Adding dilute hydrochloric acid to copper oxide.
(c) Heating copper carbonate.
(d) Passing oxygen over heated copper oxide.
(iv) The metallic oxide which cannot be reduced by normal reducing agents is:
(a) Copper (II) oxide
(b) Zinc oxide
(c) Magnessium oxide
(d) Iron (III) oxide
(v) The number of atoms in 192g of sulphur are: (S = 32)
(a) 0.75 x 6.023 x 1023
(b) 6.0 x 6.023 x 1023
(c) 3.0 x 6.023 x 1023
(d) 3.5 x 6.023 x 1023
(b) State your observation for the following reactions: [5]
(i) When ammonia gas is passed through hydrochloride acid.
(ii) Molten lead bromide is electrolyzed using graphite electrodes.
(iii) Ammonia is brunt in air.
(iv) Sodium hydroxide solution is added to zinc sulphate solution till it is in excess.
(v) Nitric acid is treated with freshly prepared acidified ferrous sulphate solution.
(c) From the list of substances given in the brackets choose the substance only once which fits the description given below: [5]
[Lead chloride, Zinc hydroxide, Lead hydroxide, Sliver chloride, Copper hydroxide, Magnesium hydroxide]
(i) A metal chloride soluble in ammonium hydroxide
(ii) A metal hydroxide which is soluble in sodium hydroxide solution only
(iii) A metal hydroxide which is soluble in ammonium hydroxide solution only
(iv) A metal chloride soluble in hot water
(v) A metal hydroxide insoluble in sodium hydroxide as well as ammonium hydroxide
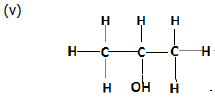
(d) Give IUPAC name of the following organic compounds:

(e) Write balanced chemical equation for the following reactions: [5]
(i) Copper metal reacts with dilute nitric acid.
(ii) Sodium aluminate undergoes hydrolysis at 500C.
(iii) Alkyl halide undergoes hydrolysis with aqueous alkali solution.
(iv) Dehydration of ethyl alcohol at 3500C.
(v) Sulphur is treated with concentrated sulphuric acid.
(f) Give appropriate scientific reasons for the following statements: [5]
(i) Although copper is a good conductor of electricity it is not an electrolyte.
(ii) Methane does not undergo addition reaction but ethene does.
(iii) Caustic soda is added to bauxite ore during the process of concentration of ore.
(iv) The blue colour of aqueous copper sulphate does not change when it is electrolyzed using copper electrode.
(v) Direct absorption of HCI gas in water is not preferred.
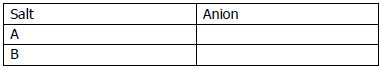
(g) Salts A, B, C, D and E undergo reaction (i) to (v) respectively. Identify the anion present in these salts on the basis of these reactions. Tabulate your answers in the format given below. [5]
(i) When silver nitrate solution is added to a solution of A, a white precipitate, insoluble in nitric acid, is formed.
(ii) Addition of dilute hydrochloric acid to B produces a gas which turns lead acetate paper black.
(iii) When dilute sulphuric acid added to C a gas is produced which turns acidified potassium dichromate solution from orange to clear green.
(iv) When concentrated sulphuric acid and copper turnings are added to salt D, it liberates a gas which turns moist potassium iodide paper brown.
(v) Addition of dilute hydrochloric acid to E produces effervescence. The gas produced turns lime water milky but does not affect acidified potassium dichromate.

(h) (i) A gas jar contains 7.2 x 1020 molecules of ammonia gas. Find [3]
(a) number of moles
(b) weight in grams
(c) volume in cm3 of ammonia gas at S.T.P. [N= 14, H = 1]
(ii) Calculate the percentage by weight of chromium in potassium dichromate.
[K = 39, Cr = 52, O = 16]
Sample Paper ICSE Class 10 Chemistry Set H
SECTION II (40 Marks)
Attempt any four questions from this section)
Question 2
(a) From the given diagram of electrolytic cell used in extraction of aluminium, answer the following questions:
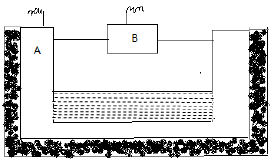
(i) Identify A and B and write what they are made of.
(ii) What is the composition of C in correct proportion?
(iii) Write the reaction that occurs at B.
(iv) Why is layer of powdered coke sprinkled over the electrolytic mixture?
(v) Mention any one difficulty faced during electrolytic reduction of the electrolyte used.
(b) Draw the structure of the following organic compound: [3]
(i) 3,3 –dimethyl pen-1-yne
(ii) Butan-2-ol
(iii) Acetylene
(c) (i) What is a lone pair? [2]
(ii) Name the electrolyte used in electroplating with silver.
Question 3
(a) With reference to the first three periods of the modern periodic table, answer the following questions given below. [5]
(i) Write the chemical formula of the nitride of the element with atomic number 12.
(ii) Name the element with highest ionization potential.
(iii) State the electronic configuration of the elements in the third period which gains one electron to form an anion.
(iv) How does the trend of electronegativity vary as we move down group 17.
(v) Name the element with largest atomic size.
(b) 200ml of ethane is burnt in air (containing 20% oxygen) as per the equation – C2H4 + 3O2 → 2CO2 + 2H2O. Calculate the resultant mixture composition [at 1000C and constant pressure] [3]
(c) Define isomerism. Draw structure of any two isomer of butene. [2]
Question 4
(a) The questions given below are related to the preparation of ammonia in laboratory: [5]
(i) Give a balanced chemical reaction for the laboratory preparation of ammonia using ammonium sulphate and slaked lime.
(ii) Name the method used for collecting ammonia.
(iii) Why is ammonium nitrate not used in the laboratory preparation of ammonia?
(iv) Name the drying agent which is not used to dry ammonia gas.
(v) State your observation when ammonia reacts with excess of greenish yellow gas.
(b) P + 5HNO3 → H3PO4 + H2O + 5NO2. If 9.3 grams of phosphorous was used in the reaction. Calculate. [3]
(i) Number of moles of phosphorous taken.
(ii) The mass of phosphoric acid formed.
(iii) The volume of NO2 produced at S.T.P [H = 1, N = 14, P = 31, O = 16]
(c) Compound A, when warmed with cone sulphuric acid gives a gas which fumes in moist air and gives dense white fumes with ammonia. [2]
(i) Name the anion present in the compound A.
(ii) Write the equation for the laboratory preparation of the above evolved gas.
Question 5
(a) A compound X consists of 4.8% carbon and 95.2% bromine by mass.
(i) Determine the empirical formula of this compound working correct to one decimal place (C = 12; Br = 80) [3]
(ii) If the vapour density of the compound is 252, what is the molecular formula of the compound. [1]
(iii) Name the type of chemical reaction by which X can be prepared from ethane.[1]
(b) Give a chemical test to distinguish between the following: [3]
(i) Lead nitrate and Lead chloride
(ii) Ferrous sulphate and Copper sulphate
(iii) Ethane and Ethene
(c) Give the composition of the following alloys: [2]
(i) Brass
(ii) Duralumin
Question 6
(a) An amorphous white salt on strong heating gave a gas which turns lime water milky and a residue is left which turned yellow when hot and white when cold. The residue is dissolved in dil. Sulphuric acid by heating to form a clear solution. To this solution is then added sodium hydroxide solution slowly when a white ppt is first formed which dissolved completely on excess of sodium hydroxide. Based on this answer the following questions: [5]
(i) Name the cation and anion present in the amorphous white salt.
(ii) Name the residue formed.
(iii) Write the chemical equation between the residue and dil. Sulphuric acid.
(iv) Identify the white ppt and give the chemical equation when the precipitate dissolved in excess of ammonia solution.
(b) 21.2 g of an impure mixture containing anhydrous sodium sulphate is dissolved in water. An excess of barium chloride solution is added when 1.74g of barium sulphate is obtained as a dry precipitate. Calculate the percentage purity of the impure sample. [Na = 23, S = 32, O = 16, Ba = 137]
(c) What is the principle behind the froth floatation method? What kind of ores are concentrated by this process? [2]
Question 7
(a) Give balanced chemical reaction for the following conversions:
(i) Lead nitrate A Lead oxide B Nitrogen
→ → C↑
Ammonia
(ii) Iron D Ferrous chloride E Ferrous carbonate
→ →
(b) The following questions are related to electrolysis of acidulated water: [3]
(i) Electrolysis of water is carried out by adding traces of dilute sulphuric acid. Justify.
(ii) Write the equation for the reaction that takes places at anode and cathode.
(c) Define the following: [2]
(i) Lone pair
(ii) Catenation