Acids Bases and Salts class 10 important questions are one of the best materials that students can get, as it will help them to concentrate better and reduce the level of stress that students face during the furious year.
Important Questions are essential for conceptual understanding and scoring good marks, and for Revision, Important Questions are always considered as the best when your exams are coming. We give ICSE Important Questions on acids bases and salts class 10 in a straightforward, free downloadable PDF design for the students to figure out a better understanding of the topics.
Students of ICSE Class 10 should refer to Acids, Bases And Salts ICSE Class 10 Chemistry Questions below which have come in past board exams. You should always go through questions that have come in previous years. This will help you to understand the pattern of questions in ICSE Class 10 Chemistry and prepare accordingly. This will help you to get better marks in ICSE Class 10 Board Exams
ICSE Class 10 Chemistry Important Questions Acids, Bases And Salts
Acids, Bases And Salts is an important chapter in ICSE Class 10 Chemistry. Our faculty has prepared the following ICSE Class 10 Chemistry Questions and answers based on the latest syllabus and books issued for the current academic year. Please refer to the solved questions below and also see links provided for other chapters.
Acids, Bases And Salts ICSE Class 10 Chemistry Questions
Acids, Bases And Salts ICSE Class 10 Chemistry Questions
Introduction
Acids Bases and Salts
➢ Acid is derived from the latin word acidus meaning ‘sour’ as it is associated with ‘sour’ taste of the fruits.
➢ Metallic oxides and hydroxides form the group called Bases.
➢ A compound formed by partial or complete replacement of one or more Hydrogen ion of an acid by metallic ions is called Salt.
➢ Acids and bases were first identified as specific types of compounds because of their behavior in aqueous solutions.
An Acid is a substance that produces H3O+ (H+) when it is dissolved in water. It is a proton donor and an electron pair acceptor or a species that donates protons. For example : HCl, H2SO4, CH3COOH.
A Base is a substance that produces an hydroxyl ion (OH–) when it is dissolved in water (Arrhenius), a proton acceptor (Brønsted), or an electron donor. For example : NaOH, KOH, NH3.
A Salt is an ionic compound that dissociates to yield a positive ion other than hydrogen ion (H+) and a negative ion other than hydroxyl ion (OH–). For example: NaCl, KCl, Na2SO4, KHCO3.
➢ Antacid indigestion tablets are mild alkalis that react by neutralizing excess stomach acid which is the ‘strong’ hydrochloric acid.
➢ Acidic bee stings can be soothed, i.e. neutralized by calamine lotion, which is a mild alkali based on zinc oxide and you can also use baking soda.
➢ Alkaline wasp stings can be neutralized with vinegar that is a weak acid.
1. Define acid.
Ans. An acid is a compound which when dissolved in water yields H+ as the only positively charged ion producing H3O+ ions.
The presence of H+ is responsible for the characteristic properties of the acid
Ex : Hydrochloric acid (HCl) gives H+(aq) and Cl–(aq) ions, sulphuric acid (H2SO4) gives 2H+(aq) and SO42–(aq) ions and nitric acid (HNO3) gives H+(aq) and NO3–(aq) ions.
2. With respect to the theory of ionization, define an acid.
Ans. An acid is a compound which when dissolved in water yields H+ as the only positively charged ion.
The presence of H+ is responsible for the characteristic properties of the acid.
An acid when put in water dissociates as follows:
HCl → H+ + Cl–
The proton formed then combines with water molecule to form a hydronium ion (H3O+)
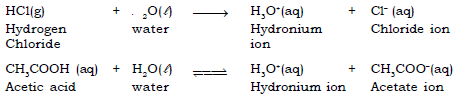
3. Mention the different ways by which acids are classified?
Ans. Acids can be classified on the basis of
a. Strength of the acid.
b. Concentration of the acid
c. Composition of the acid
d. Basicity of the Acid
e . Source of the acid
4. What do you understand by strength of an acid? On which factor does the strength of an acid depend?
Ans. Strength of acid is the hydronium ion concentration in the solution. More the number of H+ in the solution, greater is the strength of the acid. Thus the strength of an acid depends upon the degree of dissociation of the acid.
5. How are acids classified on the basis of their strength?
Ans. On the basis of strength, acids can be classified as :
Strong acid : An acid which dissociates almost completely in aqueous solution thereby producing a large concentration of H+ ions.
Eg : HCl, HNO3 etc.

Weak acid – An acid which dissociates partially in aqueous solution thereby producing a low concentration of H+. Most molecules remain undissociated in solution.
Eg : CH3COOH, HCOOH, H2CO3 etc.

6. What do you understand by concentration of acid ?
Ans. Concentration of an acid means the amount of acid present in a definite amount of its aqueous solution.
7. How are acids classified on the basis of their concentration?
Ans. Concentrated acid : An acidic solution in which there is a relatively high percentage of the acid is classified as a concentrated acid.
E.g. Concentrated hydrochloric acid (HCl), concentrated sulphuric acid (H2SO4), concentrated nitric acid (HNO3), concentrated acetic acid (CH3COOH) etc.
Dilute acid : An acidic solution in which there is a relatively low percentage of the acid, is classified as a dilute acid.
E.g. Dilute hydrochloric acid, dilute sulphuric acid, dilute nitric acid, dilute acetic acid etc.
8. What are Oxy – acids?
Ans. Oxy – acids : Oxy – acids are those, which contain oxygen in their composition along with hydrogen and some other element.
E.g. : Sulphuric acid (H2SO4), Oxalic acid (H2C2O4) Nitric acid (HNO3), Acetic acid (CH3COOH).
9. What are Hydracids?
Ans. Hydracids : Acids, which contain hydrogen together with other elements, except oxygen, in their composition, are called Hydracids.
E.g. : Hydrochloric acid (HCl), Hydroiodic acid (HI), Hydrobromic acid (HBr).
10. Define Basicity of an acid.
Ans. Basicity of an acid is the number of hydrogen ions (H+) which can be produced per molecule of the acid in aqueous solution or the number of hydroxyl ion with which one molecule of an acid combines.
11. How are acids classified on the basis of their Basicity?
Ans. On the basis of the Basicity acids are classified as :
Monobasic acid : A monobasic acid has one replaceable hydrogen ion.
Hence they produce only one H+ ion in solution. Hence these acids combine with one hydroxyl group of a base to form salt and water.
E.g. : Formic acid (HCOOH), Nitric acid (HNO3), Hydrochloric acid (HCl), Acetic acid (CH3COOH)
Dibasic acid : A dibasic acid has 2 replaceable hydrogen ions. Hence they produce 2 H+ ions in solution. Hence these acids combine with 2 hydroxyl group of a base to form salt and water.
Example : Sulphuric acid (H2SO4) dissociates in two steps in water.

E.g. : Sulphuric acid (H2SO4), Carbonic acid (H2CO3), Oxalic acid : (COOH)2
Tribasic acids : A tribasic acid has 3 replaceable hydrogen ions. Hence they produce 3 H+ ions in solution. Hence these acids combine with 3 hydroxyl group of a base to form salt and water.
E.g. : Phosphoric acid (H3PO4).
Phosphoric acid dissociates in water in three steps.

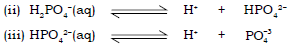
12. How are acids classified on the basis of their source.
Ans. Acids that are derived from plants are called organic acids.
E.g. Citric acid, tartaric acid etc.
Acids that are derived from minerals are called mineral acids.
E.g. Nitric acid, hydrochloric acid etc.
13. Give the balanced chemical equations of the following reactions with respect to preparation of acids.
1) Preparation of hydrogen chloride by direct synthesis.
Ans. H2 + Cl2 → 2HCl
2) Combination of Bromine and hydrogen to produce an acid
Ans. H2 + Br2 → 2HBr
3) Preparation of hydrogen iodide using iodine
Ans. H2 + I2 → 2HI
4) Preparation of sulphuric acid from its acidic oxide
Ans. SO3 + H2O → H2SO4
5) Preparation of carbonic acid from its acid anhydride.
Ans. CO2 + H2O → H2CO3
6) Addition of Sulphur dioxide to water
Ans. SO2 + H2O → H2SO3
7) Preparation of phosphoric acid from phosphorous pentoxide.
Ans. P2O5 + 3H2O → 2H3PO4
8) Preparation of nitric acid using a non-volatile acid
Ans.
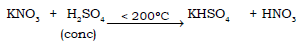
9) Action of conc sulphuric acid on sodium chloride at a temperature of less than 200ºC.
Ans.
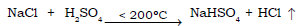
10) Preparation of a non-volatile acid using a volatile acid. OR preparation of sulphuric acid by oxidation of Sulphur
Ans. S + 6HNO3 → H2SO4 + 2H2O + 6NO2 ↑
14. Give the Physical properties of dilute Acids.
Ans. The physical properties of dilute acids are as stated under :
a. Acids are sour to taste.
b. Acids are corrosive in nature.
c. Litmus turns from blue to red.
d. Phenolphthalein is colourless & remains colourless in acidic medium.
e . Methyl Orange turns from orange to pink in acidic medium.
f. Physical state : Some acids are solids and some acids are liquids.
15. Give the balanced chemical equations for the following reactions with respect to chemical properties of acid.
Ans.
(i) Action of dilute HCl on zinc
Zn + 2HCl → ZnCl2 + H2 ↑
(ii) Preparation of magnesium chloride using dil HCl
Mg + 2HCl → MgCl2 + H2 ↑
(iii) Action of cold dilute HNO3 on Mg
Mg + 2HNO3 → Mg(NO3)2 + H2 ↑
1% dilute
cold
(iv) Preparation of sodium sulphate by neutralization of sodium hydroxide
2NaOH + H2SO4 → Na2SO4 + 2H2O
(v) Conversion of copper oxide to copper chloride
CuO + 2HCl → CuCl2 + H2O
(vi) Decomposition of sodium carbonate by hydrochloric acid.
Na2CO3 + 2HCl →2NaCl + H2O + CO2 ↑
(vii) Action of dilute sulphuric acid on potassium bisulphite
2KHSO3 + H2SO4 → K2SO4 + 2H2O + 2SO2 ↑
(viii) Reaction of dilute hydrochloric acid and sodium sulphide
Na2S + 2HCl → 2NaCl + H2S ↑
16. Give the uses of acids.
Ans. The uses of acids are as follows :
a. As eyewash – Boric acid
b. As a food preservative – Citric acid
c. In flavoring drinks – Carbonic acids
d. Ink stain remover – oxalic acid
e . Baking powder – tartaric acid
f. Cooking – acetic acid
g. Pickling of metals – Hydrochloric acid
17.(a) Define base.
Ans. Base is a compound which reacts with hydronium ions of an acid to give salt and water only.
Eg: CuO + 2HCl → CuCl2 + H2O
CuC0 + N2 + H2SO4 → CuSO4 + 2H2O
(b) What is a basic oxide ?
Ans. A basic oxide is a metallic oxide which contains the anion O2- and reacts with an acid to form salt and water only. Eg : Na2O, CaO, CuO.
(c) What is a basic hydroxide ?
Ans. It is a metallic hydroxide which contains OH– and will react with an acid to form salt and water only. e.g. NaOH, Al(OH)3
18. With respect to the theory of ionization, define a base.
Ans. Base is a compound which when dissolved in water yields OH– as the only negatively charged ion. The presence of OH– is responsible for the characteristic properties of the base.
A base when put in water dissociates as follows :

19. What is an alkali? How do they differ from bases? Give few examples.
Ans. All alkalis, are bases that are soluble in water and yield hydroxyl ion (OH–) as the only negative ions. Sodium hydroxide (NaOH), potassium hydroxide (KOH), calcium hydroxide Ca(OH)2 and ammonium hydroxide (NH4OH) are
the common alkalis.
All alkalis are bases but all bases are not alkali.
For e.g. NaOH is an alkali as it is soluble in water but Cu(OH)2 is not an alkali as it is insoluble in water. However both are bases as they contain the OH– group.
20. How are bases classified?
Ans. Bases can be classified on the basis of :
a. Strength of the base.
b. Concentration of the base
c. Acidity of the base
21. What do you understand by strength of a base? On which factor does strength of a base depend on?
Ans. Strength of base is the hydroxyl ion concentration in the solution. More the number of OH– ion in the solution greater is the strength of the base Thus the strength of the base depends upon the degree of dissociation of the base.
22. How are bases classified on the basis of their strength?
Ans. On the basis of strength, bases can be classified as : Strong base : A base which dissociates almost completely in aqueous solution thereby producing a large concentration of OH– ions.
E.g. : NaOH, KOH etc
Weak base : A base which dissociates partially in aqueous solution thereby producing a low concentration of OH–. Most molecules remain undissociated in solution.
E.g. : NH4OH
23. How are bases classified on the basis of their concentration?
Ans. On the basis of concentration, base can be classified as :
Concentrated base : A basic solution in which there is a relatively high percentage of the base is classified as a concentrated base.
E.g. Concentrated Sodium hydroxide, Concentrated Potassium hydroxide etc.
Dilute base : A basic solution in which there is a relatively low percentage of the base, is classified as a dilute base.
E.g. dilute sodium hydroxide, dilute potassium hydroxide etc.
24. Define Acidity of the base :
(a) Monoacidic base : A monoacidic base has one replaceable hydroxyl group. Hence they produce only one OH– ion in solution. Hence these bases combine with one hydrogen ion of an acid to form salt and water.
E.g. : Sodium hydroxide (NaOH), Potassium hydroxide (KOH)
(b) Diacidic base : A diacidic base has 2 replaceable hydroxyl groups. Hence they produce 2OH– ions in solution. Hence these bases combine with 2 hydrogen ions of an acid to form salt and water.
E.g. : Iron (II) hydroxide [Fe(OH)2], Magnesium hydroxide [Mg(OH)2], Copper hydroxide [Cu(OH)2]
(c) Triacidic base : A Triacidic base has 3 replaceable hydroxyl groups. Hence they produce 3OH– ions in solution. Hence these bases combine with 3 hydrogen ions of an acid to form salt and water.
E.g. : Iron (III) hydroxide [Fe(OH)3], Aluminium hydroxide [Al(OH)3]
25. Give balanced chemical equations for the following reactions with respect to preparations of basis :
a) Preparation of sodium oxide from sodium
Ans. 4Na + O2 → 2Na2O
b) Burning of magnesium to produce magnesium oxide
Ans. 2Mg + O2 → 2MgO
c) Preparation of Iron (III) oxide from iron
Ans. 4Fe + 3O2 → 2Fe2O3
d) Preparation of sodium hydroxide from its basic oxide.
Ans. Na2O + H2O → 2NaOH.
e) Dissolution of potassium oxide in water
Ans. K2O + H2O → 2KOH
f) Preparation of ammonium hydroxide from ammonia.
Ans. NH3 + H2O → NH4OH
g) Action of cold water on potassium
Ans. 2K + 2H2O → 2KOH + H2 ↑
h) Preparation of sodium hydroxide from sodium
Ans. 2Na + 2H2O → 2NaOH + H2 ↑
i) Preparation of a metallic hydroxide using calcium
Ans. Ca + 2H2O → Ca (OH)2 + H2 ↑
j) Conversion of copper carbonate to copper oxide
Ans.
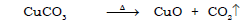
k) Decomposition of magnesium carbonate
Ans.
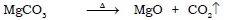
l) Preparation of zinc oxide from zinc carbonate
Ans.

m) Action of heat on calcium nitrate.
Ans.
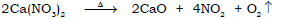
n) Preparation of magnesium oxide from magnesium nitrate.
Ans.

o) Preparation of copper hydroxide from copper sulphate
Ans. CuSO4 + 2NaOH → Cu(OH)2 ↓ + Na2SO4
p) Conversion of FeCl3 to Fe(OH)3
Ans. FeCl3 + 3NaOH / 3NH4OH → Fe(OH)3 ↓ + 3NaCl / 3NHCl
26. Give the physical properties of bases.
Ans. The physical properties of bases are as stated under :
a. Bases are bitter to taste.
b. They are soapy and slippery to touch.
c. Litmus turns from red to blue.
d. Strong alkalis like NaOH and KOH are highly corrosive in nature. It corrodes the organic tissues like skin.
27. Give balanced chemical equations for the following reactions with respect to chemical properties of bases.
Ans. a) Action of calcium hydroxide on ammonium chloride.
Ca(OH)2 + 2NH4Cl → CaCl2 + 2H2O + 2NH3
b) Neutralization of lead oxide with nitric acid
PbO + 2HNO3 → Pb(NO3)2 + H2O
c) Preparation of calcium acetate from calcium hydroxide
Ca(OH)2 + 2CH3COOH → (CH3COO)2Ca + 2H2O
d) Reaction of sodium hydroxide with copper chloride
CuCl2 + 2NaOH → 2NaCl + Cu(OH)2↓
e ) Precipitation of zinc hydroxide from zinc sulphate
ZnSO4 + 2NaOH → Na2SO4 + Zn(OH)2↓
28. Give the uses of bases.
Ans. Some important bases and their uses :
a. Manufacture of soap : NaOH – Caustic soda.
b. Manufacture of bleaching powder : Ca(OH)2 – slaked lime
c. Antacid : Mg(OH)2
d. Fire extinguisher : Al(OH)3
e . Grease stain removers : NH4OH
29. What do you mean by Amphoteric oxides or hydroxides ?
Ans. Certain metallic oxides and hydroxides react with both acids as well as with alkali to form salt and water. These oxides or hydroxides are called Amphoteric oxides or hydroxides.
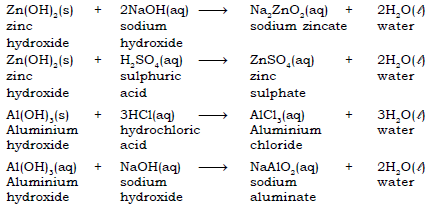
30. Give a brief account of the modern concept or Lowry Bronsted of acids and bases.
Ans. When an acid is dissolved in water it yields H+ which is a proton.
Therefore an acid can also be defined as a Proton donor while a base is defined as a proton acceptor. This is known as Lowry Bronsted’s theory of acids and bases.
31. What is neutralization? OR Define the term neutralization.
Ans. When the H+ ion from the acid combines with the OH– of the base to give
salt and water molecule, the process is termed as neutralization.

32. What do you mean by heat of neutralization?
Ans. Heat of neutralization is defined as the amount of heat liberated when 1 gram equivalent of an acid or a base gets completely neutralized.
33. What does term pH express?
Ans. The term pH expresses the strength of an acid or base. Thus pH expresses the concentration of H+ or OH– ions in solution of an acid or the base.
34. Define pH.
Ans. The pH is defined as: ‘the negative logarithm to the base 10 of the hydrogen ion concentration, expressed in moles per litre’.

35. What is pH Scale? What is the acidic, alkaline and neutral range in pH Scale?
Ans. pH Scale measures the acidity or basicity of a solution in terms of the hydronium ion concentration. The pH scale ranges from 0 to 14.
A pH of 7 means it is a neutral solution. Pure water has a pH of 7. A pH of less than 7 means the solution is acidic. A pH of more than 7 means the solution is basic.
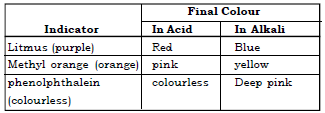
36. What are indicators?
Ans. Indicators are weak organic compounds which change colour in accordance to the pH of the solution & help to determine whether solution is acidic, alkaline or neutral.
37. Name the indicators used to identify the Acidity, Basicity or Neutrality of a solution.
OR Name the common acid – base indicators.
Ans. The common indicators used are litmus, methyl orange and phenolphthalein.
The colour of indicators change sharply as the pH of the medium changes.
However, it does not give a specific idea of the pH of the solution.
38. What is an universal indicator? How are they better than ordinary indicators?
Ans.

A Universal indicator, Eg. – pH strip is an indicator containing mixture of organic dyes, it not only shows whether the solution is acidic or basic, but also shows the approximate pH values by giving a wider range of colours for different values of pH.
39. Give examples of universal indicator.
Ans. pH strip or pH solution.
40. What is litmus? How is litmus solution prepared?
Ans. Litmus is a natural purple coloured indicator obtained from lichens. It is prepared by dissolving 0.5 g of litmus in 1 litre of distilled water and then filtering it to obtain the indicator.
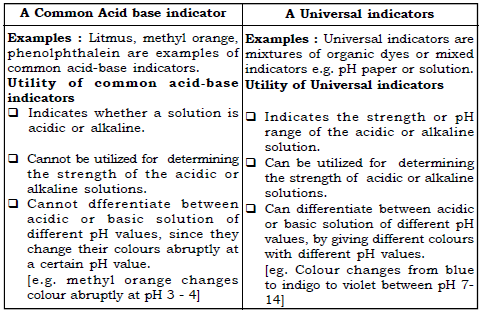
41. Universal indicator is better than a common acid base indicator. Explain.
Ans. A common acid base indicator
➢ will only tell whether the solution is acidic, alkaline or neutral.
➢ cannot be used to determine the strength of the solution.
➢ show colour changes abruptly for certain values of pH.
Whereas, a universal indicator gives a range or a spectrum depending upon the strength of the acid or the base. Thus it can be utilized to determine the strength of the solution by changes in its colour accordingly.
42. Mention the uses of pH indicators.
Ans. Uses of pH indicators are as stated below :
a. Used to determine acidity or alkalinity of the solution in agriculture for better growth and yield of crops.
b. Helps to determine pH of milk. pH below 6 indicates that the milk is sour and thus help in dairies.
c. Helps to test urine and blood, to test for various diseases.
43. Define salt?
Ans. Salt is a compound, which on dissociation in water yields positive ions other than a hydrogen ion or hydronium ion, and a negative ion other than hydroxyl ion.
Eg. NaCl(aq) → Na+ (aq) + Cl– (aq)
OR A salt is a compound formed by partial or complete replacement of the replaceable hydrogen ion of an acid by a metallic ion or ammonium ion (basic radical)
44. Give the classification of salts.
Ans. Salts can be classified as follows :
a. Normal salt b. Acid salt e . Double salt
c. Basic salt d. Complex salt f. Mixed salt
45. Define each of the above types of salts.
Ans. a. Normal salt : A salt which does not have any replaceable hydrogen ions is a normal salt.
It is obtained by replacing all the hydrogen ions of an acid by metal ions or ammonium ions.
Examples : NaCl, Na2SO4, Na3PO4, NH4Cl, KCl.
b. Acid salts : A bisalt or an acid salt contains replaceable hydrogen ions in association with the radicals.
It is obtained by the incomplete or partial replacement of hydrogen ions by metal ions or ammonium ions i.e. a BASIC RADICAL.
Examples : NaHCO3, NaH2PO4 and Na2HPO4.
These salts contain H+ which dissociate in solution thereby imparting
properties of acid.
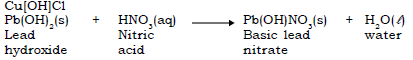
c. Basic Salts : A basic salt may also be formed by the partial replacement of the hydroxyl groups of a diacidic or triacidic base, by an acid radical. Here the proportion of base is much higher in proportion to the acid
Example : Basic copper nitrate Cu[OH]NO3, Basic Copper chloride
d. Complex Salts : Salts are formed by mixing solutions of simple salts followed by crystallization.
Salts that dissociate in water to give one simple ion and one complex ion are called complex salts.
Examples : Potassium mercuric iodide, Sodium silver cyanide, Sodium zincate etc.,
e. Double salt : It is a salt which contains a mixture of two simple salts chemically combined. It is a salt formed by mixing saturated solutions of two simple salts followed by crystallisation of the saturated solution.
Example : Alum : K2SO4. Al2(SO4)3. 24H2O
Mohr’s salt (Ferrous ammonium sulphate)
Potash Alum (NH4)2SO4.FeSO4.6H2O
f. Mixed salt : It is a salt which has two or more basic radicals or acid radicals.
Example : Sodium potassium carbonate – NaKCO3
Na+ and K+ – Two basic radicals.
46. Define the following. Give examples of each.
Ans. a. Water of Crystallisation: The number of water molecules that enter in a lose chemical combination with one molecule of the salt on crystallisation from its aqueous solution. The water of crystallisation is responsible for colour and shape of the crystals.
Example : Hydrous Copper sulphate [CuSO4.5H2O] contains five molecules of water and is blue in colour.
On losing water of crystallisation it turns into white anhydrous powder. On adding water, white anhydrous copper sulphate regains its colour.
b. Hydrated salts : The salts which contain the water of crystallisation in their molecules are called Hydrated salts.
Examples

c. Efflorescence : It is the property by which crystalline hydrated salts when exposed to air, lose their water of crystallisation, fully or partially, and crumble into a powder. Such substances are termed as efflorescent substances.
Examples
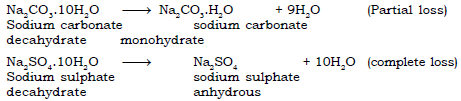
d. Deliquescence : Certain water soluble salts which when exposed to the atmosphere absorb water from the atmosphere dissolve in it and thus change its state to liquid. The process is called deliquescence and the substances are called deliquescent substances.
Examples
e. Hygroscopic substances: Certain compounds which when exposed to the atmosphere absorb water from the atmosphere and do not dissolve in it.
Example : Sodium Chloride (NaCl), Sulphuric acid (H2SO4)
47. Name some crystalline salts which have no water of crystallization in their molecule?
Ans. KCl, NaCl, AgI, Pb(NO3)2, (NH4)2SO4, KNO3
48. Give the effect of heat on hydrous salts.
Ans. On heating, hydrous crystals lose their water of crystallization and turn into a colourless powder. They are then said to be anhydrous.
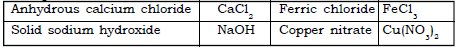
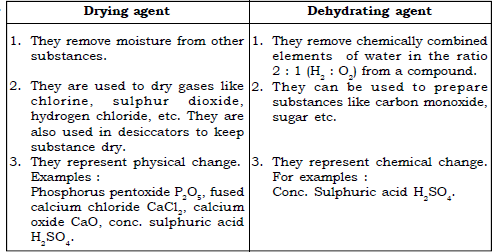
49. What are drying agents?
Ans. Substances that absorb or take away the moisture from a substance are drying agents.
Examples : anhydrous calcium chloride, quick lime and concentrated sulphuric acid are used as drying agents.
50. Give the balanced chemical equation with respect of the preparation of salts.

51. Give a brief account of the preparation of FeCl3 by Direct synthesis.
Ans. Preparation of Ferric chloride : Dry chlorine is passed through a combustion tube containing a coil of heated iron wire. A red glow appears with evolution of heat and light. It is an exothermic reaction & hence only initial heating is required. Reddish brown vapours are obtained which is led into a flask surrounded by freezing mixture (ice + salt).
52. Why is the apparatus for preparation of FeCl3 attached to desiccators?
Ans. Ferric chloride is a deliquescent salt. It absorbs the moisture from the air and turns into a saturated solution. Hence to keep it in a solid state, it has to be kept in desiccators or air tight bottles.
53. What do you understand by hydrolysis of salts?
Ans. Hydrolysis of salts are reactions of salts with water resulting in formation of a solution which is either acidic, basic or neutral.
54. Give examples of :
Ans. a. Salt of strong acid strong base : Sodium chloride, potassium chloride.
b. Salt of strong acid weak base : Ammonium chloride.
c. Salt of weak acid weak base : Ammonium carbonate.
d. Salt of weak acid strong base : Potassium carbonate.
55. Give the hydrolysis of the following :
a. Sodium bicarbonate, b. Ammonium chloride
and state the colour change in litmus observed in the salt solution.
Ans. a. Sodium bicarbonate : Sodium bicarbonate is a salt of weak acid and strong base.
NaHCO3 + H2O → NaOH + H2CO3
Therefore the solution will be slightly alkaline and red litmus paper will turn blue.
b. Ammonium chloride : This is a a salt of strong acid and weak base
NH4Cl + H2O → NH4OH + HCl
Therefore the solution will be slightly acidic and blue litmus paper will turn red.
56. Explain the term ‘acid rain’ and state its composition. Give balanced equations for formation of (i) Sulphuric acid (ii) Nitric acid – in acid rain.
Ans. Acid rain refers to rain which is acidic in nature. It is generally a complex mixture of sulphuric acid [H2SO4] along with sulphurous acid [H2SO3] & nitric acid [HNO3] along with nitrous acid [HNO2].
(i) Formation of sulphuric acid

(ii) Formation of Nitric acid

Memory charts
An important rule is :
Metal oxides are basic : when soluble, metal oxides form alkaline solutions in water.
Non-metal oxides are acidic : when soluble, non-metal oxides form acidic solutions in water.
METALS :
(i) Form basic oxides which react with acids to form salts, if oxide is soluble in water an alkali is formed and turn litmus blue;
(ii) React with acids to form a salt solution and hydrogen gas,
(iii) Readily combine with non-metals to form ionic compounds.
NON-METALS :
(i) Form acidic oxides that react with alkalis to form salts, if oxide soluble in water it will turn litmus red;
(ii) Do not usually react with acids,
(iii) Readily combine with metals to form ionic compounds,
(iv) Combine with other non-metals to form covalent compounds.
You must know four general word equations, all of which involve neutralisations (students would be expected to interpret them as symbol equations too)
(i) Metal + acid → a salt + hydrogen
(ii) Basic oxide or hydroxide + acid → a salt + water
(iii) Carbonate + acid → a salt + water + carbon dioxide
(iv) Ammonia + acid → ammonium salt
ADDITIONAL QUESTIONS
I. Name the following :
(a) A basic solution which does not contain a metallic element.
Ans. Ammonium hydroxide.
(b) An alkali which on dissociation produces a high concentration of hydroxyl ions.
Ans. Sodium hydroxide.
(c) A complex salt solution used for testing a basic gas lighter than air.
Ans. Nessler’s reagent, K2HgI4
(d) An alkali which reacts with hydrochloric acid to give a salt which on hydrolysis gives a slightly acidic solution.
Ans. Ammonium / Calcium hydroxide.
(e) An ion which combines with a polar covalent molecule to form ammonium ion.
Ans. Hydrogen ion (H+)
(f) The ion other than ammonium ion formed when ammonia dissolves in water.
Ans. Hydroxyl ion (OH–)
(g) Two bases which are not alkalies.
Ans. Magnesium hydroxide Mg(OH)2, Ferrous hydroxide Fe(OH)2 or Iron (II) hydroxide.
(h) A mixed acid anhydride.
Ans. Nitrogen dioxide.
(i) A salt insoluble in cold water but soluble in hot water
Ans. Lead (II) chloride, PbCl2.
(j) Two basic oxides which are soluble in water.
Ans. Potassium oxide (K2O) and Sodium oxide (Na2O)
(k) The name of the acid salt which gives sodium ions and sulphate ions in solution.
Ans. Sodium hydrogen sulphate
(l) Strong acid containing chlorine.
Ans. Hydrochloric acid (HCl).
(m) Two dibasic acids containing sulphur.
Ans. Sulphuric acid (H2SO4) and sulphurous acid (H2SO3).
(n) Acid anhydride of sulphuric acid.
Ans. SO3 is acid anhydride of sulphuric acid.
(o) Two monobasic acids containing nitrogen.
Ans. Nitric acid (HNO3) and Nitrous acid (HNO2).
(p) A weak mineral / inorganic acid.
Ans. Carbonic acid (H2CO3)
(q) The type of acid which has only ions present in its solution.
Ans. Strong acid
(r) Two sulphates which are insoluble.
Ans. Lead sulphate, Silver sulphate, Calcium sulphate, Barium sulphate.
(s) A tribasic acid
Ans. Phosphoric acid.
II. Write balanced equation to satisfy each statement.
(a) Copper (II) oxide → Copper (II) sulphate ← copper (II) hydroxide
Ans.
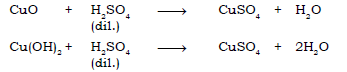
(b) Calcium oxide → Calcium chloride → Calcium carbonate
Ans.
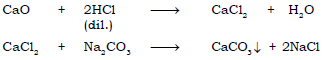
(c) Zinc sulphate ← Zn → Zinc sulphide
Ans.
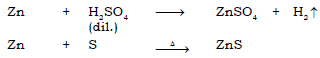
(d) Lead (II) oxide → Lead nitrate → Lead sulphate
Ans.
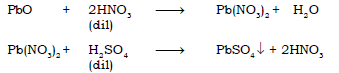
(e) Iron (II) Chloride ← Fe → Iron (III) Chloride
Ans.

III. Give one example in each case :
(a) Oxy acid – Nitric acid
(b) Hydracid – Hydrochloric acid
(c) Product obtained when lime water dries up – Calcium oxide
(d) An acid used in aerated drinks – Carbonic acid
(e) Tribasic acid – Phosphoric acid
(f) Triacidic base – Ferric hydroxide
(g) A hydrogen containing compound which is not acidic. – Methane, H2O
(h) An acid used to remove ink spots – Oxalic acid
IV. Suggest suitable method for preparation of : –
1. Which of the following methods A, B, C, D or E is generally used for preparing the chloride listed below from (i) to (v). Answer by writing down the chloride and the letter pertaining to the corresponding method. Each letter is to be
used only once.
(A) Action of an acid on a metal
(B) Action of an acid on an oxide or carbonate
(C) Direct combination
(D) Neutralisation of an alkali by an acid
(E) Precipitation (double decomposition)
(i) Copper (II) Chloride (ii) Iron (II) Chloride
(iii) Iron (III) Chloride (iv) Lead (II) Chloride
(v) Sodium Chloride
Ans. (i) Copper (II) Chloride – B
(ii) Iron (II) Chloride – A
(iii) Iron (III) Chloride – C
(iv) Lead (II) Chloride – E
(v) Sodium Chloride – D
2. The preparation of lead sulphate from lead carbonate is a two – step process. (Lead sulphate cannot be prepared by adding dilute sulphuric acid to lead carbonate). Answer the following question with respect to this process.
(a) Why is the direct addition of dilute sulphuric acid to lead carbonate an impractical method of preparing lead sulphate?
Ans. This will lead to the deposition of lead sulphate on the surface of lead carbonate which stops further reaction of sulphuric acid on it.
(b) What is the first step that is required to prepare lead sulphate from lead carbonate?
Ans. Treatment of lead carbonate with nitric acid.
(c) Write the equation for the reaction that will take place when this first step is carried out.
Ans. PbCO3 + 2HNO3 → Pb(NO3)2 + H2O + CO2 ↑
(d) Give the balanced chemical equation for the second step of the preparation
Ans. Pb(NO3)2 + Na2SO4 → PbSO4 ↓ + 2NaNO3
3. Suggest a suitable method for the preparation of salts A, B, C and D related to the descriptions given below :
(a) A is a sodium salt
Ans. By neutralisation : (titration)
(b) B is an insoluble salt
Ans. By precipitation
(c) C is a soluble salt of magnesium (using carbonate)
Ans. By the action of an acid
(d) D is a soluble salt of copper
Ans. By the action of CuO with dil. acid or Neutralisation
4. Match the Salts in column ‘A’ with the method of preparation in column ‘B’.
‘A’ ‘B’
1. Zinc sulphate (a) Precipitation
2. Ferrous sulphide (b) Neutralisation
3. Barium sulphate (c) Displacement
4. Copper sulphate (d) Titration
5. Sodium sulphate (e) Synthesis
Ans. (i) (c) Displacement
(ii) (e) Synthesis
(iii) (a) Precipitation
(iv) (b) Neutralisation
(v) (d) Titration
5. State the method only generally used for the preparation of the following salts.
(i) Zn(NO3)2 : Neutralisation
(ii) NH4Cl : Neutralisation/Titration
(iii) ZnSO4 : Simple Displacement
(iv) ZnS : Direct combination
(v) CaCO3 : Precipitation (Double decompostion)
(vi) FeCl3 : Direct combination
(vii) PbCl2 : Precipitation
(viii) Pb(NO3)2 : Neutralisation
6. How are the following salts prepared. Give balanced chemical equation
(a) Calcium sulphate (insoluble) from calcium carbonate
(b) Lead carbonate from lead nitrate
(c) Sodium nitrate from sodium hydroxide
(d) Magnesium carbonate from magnesium chloride
(e) Copper (II) sulphate from copper (II) oxide
Ans.

V. Answer the Following :
1. Write the ionisation of sulphuric acid showing the formation of hydronium ion.
Ans.

2. Classify the solution of the following as acids, bases or salts
Ammonium hydroxide, barium chloride, sodium chloride, sodium hydroxide, H2SO4 and HNO3
Ans.
Ammonium hydroxide ……………………….. Base
Barium chloride ………………………………. Salt
Sodium chloride ………………………………. Salt
Sodium hydroxide ……………………………. Base
H2SO4 ……………………………………………. Acid
HNO3 ……………………………………………. Acid
3. Two solutions X and Y have pH values of 3 and 9 respectively. Which one of these two will give a pink colour with phenolphthalein indicator?
Ans. Y, because its basic in nature.
4. Match the description below with the appropriate term from the list a to f.
a. Acidic oxide b. Alkali c. Amphoteric oxide
d. Basic oxide e . Deliquescence f. Efflorescence
(i) The property of spontaneously giving up water of crystallisation to the atmosphere.
Ans. f : Efflorescence
(ii) A compound, which is soluble in water and the only negative ions in the solution are hydroxide ions.
Ans. b : Alkali
(iii) An oxide which forms salts when it reacts with both acids and alkalies.
Ans. c : Amphoteric
5. (a) Give a balanced equation for reaction of nitrogen dioxide with water.
(b) How many types of salts does dibasic acid produce when it reacts with caustic soda solution ? Give equations.
Ans. (a) 2NO2 + H2O → HNO2 + HNO3
(b) A dibasic acid produces two types of salts acid salt & normal salt when it reacts with caustic soda solution eg. H2SO4
H2SO4 + 2NaOH → Na2SO4 + H2O
H2SO4 + NaOH → NaHSO4 + H2O
6. State giving balanced equations how would you obtain :
(i) Sulphuric acid from an acidic oxide.
Ans. By dissolving in water
SO3 + H2O → H2SO4
(ii) KOH from a basic oxide.
Ans. By dissolving in water
K2O + H2O → 2KOH
7. From the salts – anhydrous CaCl2, washing soda, CuSO4.5H2O State which compound is
(i) Efflorescent (ii) blue in colour (iii) deliquescent
Ans. (i) Washing soda (ii) CuSO4.5H2O (iii) Anhydrous CaCl2
8. Write chemical equations for the conversions as shown below with the help of diagram.

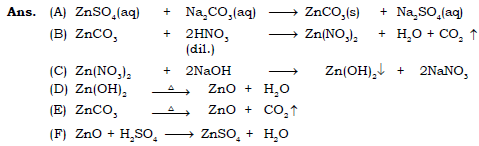
9. From the following formulae AgCl, CuCO3, CuSO4.5H2O, KNO3, NaCl, NaHSO4, Pb(NO3)2, ZnCO3, ZnSO4.7H2O
Choose one in each case having the following descriptions :
(i) an acid salt – NaHSO4
(ii) an insoluble chloride – AgCl
(iii) changes from blue to white on treating with conc. H2SO4 – CuSO4. 5H2O
(iv) changes from green to black on heating – CuCO3
(v) gives nitrogen dioxide on heating. – Pb(NO3)2
10.(a) What is the pH of pure water?
Ans. The pH of pure water is 7.
(b) What is the purpose of pH scale?
Ans. It is used to determine the acidic, alkaline or neutral nature of an aqueous solution.
(c) A is a soluble acidic oxide, B is a soluble base. Compare to pH of water what will be the pH of (i) solution A, (ii) a solution of B?
Ans. (i) pH < 7 (ii) pH > 7
11.(a) A solution has a pH of 7. Explain how you would
(i) increase its pH (ii) decrease its pH
Ans. (i) By adding some alkali like NaOH
(ii) By adding some acid like HCl
(b) If a solution changes the colour of litmus from red to blue, what can you say about its pH?
Ans. Since the solution changes the colour of litmus from red to blue, it is alkaline and hence it has pH > 7.
(c) What can you say about the pH of a solution that liberates CO2 from sodium carbonate?
Ans. Since the solution liberates CO2 from sodium carbonate, it should be acidic and has pH < 7.
12. Name the ions furnished by an acid.
Ans. It gives hydronium ions (H3O+) in water.

13. Identify which of the following terms match with the appropriate descriptions 1 to 5.
A : Hydracid B : Monobasic acid
C : Less volatile acid D : Weak acid
E : Dibasic acid F : More volatile acid
(a) An acid having basicity 1 and having only one replaceable hydrogen ion per molecule of the acid.
Ans. B
(b) An acid which dissociates to give a low concentration of H+ ions.
Ans. D
(c) An acid containing hydrogen and a non – metallic element other then oxygen.
Ans. A
(d) The type of acid which generally displaces another acid when heated with salt.
Ans. C
(e) The type of acid which reacts with a base to give an acid salt and a normal salt.
Ans. E
14. Solution P has a pH of 13, Solution Q has a pH of 6 and solution R has a pH of 2.
Which solution :
(a) will liberate ammonia from ammonium sulphate on heating ?
(b) is a strong acid
(c) contains molecules as well as ions ?
Ans. (a) Solution P
(b) Solution R
(c) Solution Q
15. Name an ion responsible for the blue colour of an aqueous solution of a salt.
Ans. Copper ion
16. Two acids A and B have pH values 1 and 5 respectively. Which is a stronger acid A or B?
Ans. A is stronger acid
17. Name the acids present in following: Orange, tamarind, apples, sour milk
Ans.Orange → Citric acid
Tamarind → Tartaric acid
Apples → Malic acid
Sour milk → Lactic acid
18. The questions (i) to (iv) are about the pH of solutions.
a) 1 b) 4 c)7 d) 10
Choose from (a) -(d) the pH of:
(i) a weakly acidic solution.
(ii) neutral.
(iii) a stronger acidic solution.
(iv) a weakly alkaline
Ans. i) b) ii) c) iii) a) iv) d)
19. Classify the following compounds into:
i) deliquescent ii) efflorescent
A. Magnesium chloride crystals
B. Zinc chloride crystals
C. Sodium carbonate crystal
D. Sodium hydroxide crystal
Ans. A. Deliquescent B. Deliquescent
C. Efflorescent D. Deliquescent
20. (a) What do you understand by this statement: “Acetic acid is a monobasic acid”?
(b) Give chemical names and chemical formula of the following compounds:
(i) Washing soda
(ii) Glauber’s salt
(iii) Epsom salt
Ans. (a) Acetic acid is a monobasic acid means it produces one hydrogen ion (H+) on complete dissociation in aqueous solution.
(b) Chemical name Chemical formula
i) Sodium carbonate Na2CO3.10H2O
ii) Sodium sulphate Na2SO4.10H2O
iii) Magnesium sulphate MgSO4.7H2O
21. The approximate pH values of certain substances are given below. Mention whether they are strongly acidic, weakly acidic, neutral, weakly alkaline or strongly alkaline.
Substance pH value
(a) Hydrochloric acid 1.0
(b) Sulphuric acid 1.2
(c) Sea water 8.5
(d) Ammonium hydroxide 11.1
(e) Sodium hydroxide 13.0
(f) Tomato 4.2
(g) Vinegar 2.8
(h) Lemon 2.3
(i) Milk 6.5
(j) Pure water 7.0
Ans. a) Strongly acidic
b) Strongly acidic
c) Weakly alkaline
d) Strongly alkaline
e ) Strongly alkaline
f) Weakly acidic
g) Strongly acidic
h) Strongly acidic
i) Weakly acidic
j) Neutral
22. (a) From the list of substances given below, name the substances which you would use to prepare each of the following salts, named in parts (i) to (iv):
Copper, lead, sodium, zinc, copper oxide, lead nitrate, sodium carbonate solution, sodium sulphate, dilute hydrochloric acid, dilute nitric acid and dilute sulphuric acid.
i) Zinc sulphate ii) Copper sulphate
iii) Sodium sulphate iv) Lead sulphate
(b) How would you obtain an acid from a non-metal? Give equation.
Ans. (a) i) For zinc sulphate: Zinc and dilute sulphuric acid
ii) For copper sulphate: Copper oxide and dilute sulphuric acid
iii) For sodium sulphate: Sodium carbonate and dilute sulphuric acid.
iv) For lead sulphate: Lead nitrate and sodium sulphate.
(b) e.g. Acid can be obtained from non-metal by direct synthesis reaction.
H2 + Cl2 → 2HCl
23. State the terms defined by the following sentences :
(a) A soluble base.
(b) The insoluble solid formed when two solutions are mixed together.
(c) An acidic solution in which there is only partial ionization of the solute molecules.
Ans. (a) Alkali
(b) Precipitate
(c) Weak Acid
VI. Choose the correct answer from the choices (i), (ii), (iii), and (iv).
(a) Tartaric acid is used:
i) in baking powder
ii) as an eyewash
iii) in cooking
iv) to remove inkstains
Answer
(i)
(b) Which of the following has water of crystallisation?
i) Zinc chloride
ii) Caustic soda
iii) Baking soda
iv) Washing soda
Answer
(iv)
(c) A normal salt:
i) contains one replaceable hydrogen atom in its molecule.
ii) exhibits the properties of an acid.
iii) does not exhibit the properties of an acid.
iv) contains three replaceable hydrogen atoms in its molecule.
Answer
(iii)
(d) If some acid is added to an aqueous solution. its pH will:
i) increase
ii) decrease
iii) remain the same
iv) first increase and then decrease
Answer
(ii)
(e) In the laboratory, lead (II) nitrate is prepared by:
i) dilute hydrogen chloride and lead(II) carbonate
ii) dilute nitric acid and lead (II) carbonate
iii) dilute sulphuric acid and lead (II) carbonate
iv) concentrated nitric acid and lead (II) carbonate
Answer
(ii)
(f) Basic calcium hydroxide is used:
i) in removing grease stains
ii) in softening water
iii) in fire extinguishers
iv) as an antacid.
Answer
(ii)
(g) An acid is called a strong acid if it:
i) ionizes almost completely
ii) reacts only on heating
iii) reacts even at room temperature
iv) none of these
Answer
(i)
VII. Fill in the blanks by choosing the correct word/ words given within brackets:
(a) A reaction between a base and an acid to produce salt and water only is called ………….. reaction.
(double decomposition/neutralization)
Answer
neutralization
(b) All nitrates of metals are ………….. in water.
(Soluble/insoluble)
Answer
Soluble
(c) Sodium chloride (NaCl) is ………….. salt.
(a hydrated/an anhydrous)
Answer
an anhydrous
(d) Sodium potassium sulphate is a …………. salt.
(complex/mixed)
Answer
mixed
(e) The number of replaceable hydroxide ions (OH–) formed by one molecule of a base in water is known as …………… .
(acidity/basicity)
Answer
acidity
(f) An example of an acid derived from a mineral is ………….. .
(citric acid / nitric acid / acetic acid)
Answer
nitric acid
(g) An example of a base which is not a alkali is …………… .
(caustic soda / zinc hydroxide / liquor ammonia / caustic potash)
Answer
zinc hydroxide
(h) A base obtained when lead nitrate undergoes thermal decomposition is ……………. .
(trilead tetroxide / lead [IV] oxide / lead [II] oxide)
Answer
lead [II] oxide
(i) An acid obtained when concentrated nitric acid is heated with sulphur is …………..
(sulphurous acid / sulphuric acid / nitrous acid)
Answer
sulphuric acid
(j) A solution whose pH is above 7 is …………..
(vinegar / milk / liquor ammonia)
Answer
liquor ammonia
(k) An example of an acid salt is ………….
(CH3COONa / NaNO3 / Na2HPO4/NaKCO3)
Answer
Na2HPO4
(l) An example of a soluble salt is ……………
(AgC1 / PbSO4 / CaSO4 / CaCl2)
Answer
CaCl2
(m) An example of an insoluble salt is ……….
[Na2CO3 / K2CO3 / MgCO3 / (NH4)2CO3)
Answer
MgCO3
(n) An example of a salt which is not a hydrated salt is …………
(blue vitriol / washing soda / sal ammoniac / epsom salt)
Answer
sal ammoniac
(o) A salt which on hydrolysis produces a neutral solution is ………….
(sodium chloride / ammonium chloride / sodium carbonate)
Answer
sodium chloride
VIII. Give reasons :
1. Hydrochloric acid is considered as a strong acid whereas acetic acid is a weak acid.
Ans. Hydrochloric acid is considered as a strong acid because it dissociates completely in water. Acetic acid is a weak acid as it dissociates partially when dissolved in water. Most of its molecules remain in molecular form in the solution.
2. It is necessary to find out the ratio of reactants required in the preparation of sodium sulphate.
Ans. The formation of sodium sulphate depends on the ratio of the reactants. If the concentration of sodium hydroxide is more than H2SO4 sodium sulphate is formed, in low concentration of sodium hydroxide, sodium bisulphate is formed.
3. Fused calcium chloride is used in the preparation of FeCl3.
Ans. Iron (III) chloride is a deliquescent salt, in order to prevent it from exposure to moisture, fused calcium chloride is used to absorb moisture in the preparation of FeCl3
4. Dry HCl gas does not change the colour of dry litmus paper.
Ans. The acidic properties of HCl is due to the presence of hydronium ion. Hydronium ion can be produced only in the presence of moisture, hence dry HCl gas does not change the colour of dry litmus paper.
5. Dil HCl acid is stronger than highly concentrated acetic acid.
Ans. HCl is a strong acid and hence dissociates completely in its solution, whereas acetic acid is a weak acid and dissociates partially in its solution. Hence dil HCl is stronger than highly concentrated acetic acid.
6. Concentrated sulphuric acid is a weaker acid compared to dilute sulphuric acid.
Ans. Strength of an acid is determined by the concentration of hydronium ions in its solution, the formation of hydronium ion depend on the amount of water. As amount of water in concentrated acid is less, concentrated sulphuric acid is a weaker acid compared to dilute sulphuric acid which has higher concentration of water than concentrated acid.
7. An aqueous solution of the salt ammonium chloride is acidic in nature while an aqueous solution of sodium chloride is neutral.
Ans. Ammonium chloride undergoes hydrolysis in its aqueous solution to form ammonium hydroxide a weak base and hydrogen chloride a strong acid, hence the solution is acidic in nature. Whereas, sodium chloride undergoes hydrolysis in its aqueous solution to produce sodium hydroxide, a strong base and hydrochloric acid a strong acid hence the solution is neutral.
8. Acetic acid does not form an acid salt but forms a normal salt.
Ans. Acetic acid has only one replaceable hydrogen ion, i.e. it is a monobasic acid hence it does not form an acid salt but forms a normal salt.
IX. What do you observe when :
1. Concentrated sulphuric acid is added to copper sulphate crystals.
Ans. Copper sulphate turns from blue to white.
2. Dilute HCl is added to a carbonate salt.
Ans. A colourless gas is released which turns lime water milky.
3. Dilute H2SO4 is added to a sulphite salt.
Ans. A colourless gas is obtained which turns acidified KMnO4 from pink to clear colourless or acidifies K2Cr2O7 orange to clear green
4. Magnesium sulphate crystals when exposed to air.
Ans. A white amorphous powder is seen on the surface.
5. Red litmus is added to aqueous solution of sodium carbonate.
Ans. Red litmus turns blue.
6. Blue litmus is added to aqueous solution of magnesium chloride.
Ans. Blue litmus turns red.
X. Distinguish between :
1. A common Acid base indicator and A Universal indicator.
Ans.
2. Acidity of bases and Basicity of acids.
Ans.

3. Acid and Alkali
Ans.

4. Drying agent and dehydrating agent
Ans.
Summary
• A salt is a compound that dissociates in water yielding positive ion other than hydrogen ion (H+) and negative ion other than hydroxyl ion (OH–).
NaCl (aq) → Na+ (aq) + Cl–(aq)
Classification of Salts
• Normal salts : NaCl, Na2SO4, etc.
• Acid salts : NaHCO3, Ca(HCO3)2, etc.
• Basic salts : Fe(OH)CO3, Fe(OH)SO4, Cu(OH)Cl, Pb(OH)NO3, etc.
• Complex salts : K2(HgI4) Na[Ag(CN)2], etc.
General Methods of Preparation of Salts
• Direct combination of metals (Synthesis) : 2Na(s) + Cl2(g) → 2NaCl(s)
• Dissolving metals in acids : Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2O(l)
• Dissolving metal oxides in acids :
ZnO(s) + 2HCl(aq) → ZnCl2(aq) + H2O(l)
• Hydroxides react with acids :
NaOH (aq) + HCl(aq) → NaCl(aq) + H2O(l)
• Bicarbonates/Carbonates, metal sulphites and metal sulphides react with acids:
PbCO3(s) + 2HNO3(aq) → Pb(NO3)2(aq) + H2O(l) + CO2(g)
FeS(s) + H2SO4(aq) → FeSO4(aq) + H2S (g)
• By precipitation, insoluble salts are prepared:
Pb(NO3)2 + H2SO4 → Pb(SO4) ↓ + 2HNO3
• Treating alkali with acidic oxides:
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
• Treating salts of volatile acid with non-volatile acid:
2NaCl(s) + H2SO4(aq) → Na2SO4(aq) + 2HCl(g)
Laboratory Preparation of Salts
• Treating metals with dilute acids; for example, zinc sulphate (ZnSO4), iron sulphate (FeSO4).
• Treating oxide or hydroxide or carbonate with acid; for example, copper sulphate (CuSO4).
• Treating alkali solution with dilute acids; for example, sodium sulphate (Na2SO4).
• Insoluble salt by precipitation; for example, CaCO3 and PbCl2.
• By direct combination; for example, iron (III) chloride (FeCl3).
• Deliquescence : Water soluble salts which on exposure to the atmosphere absorb moisture from atmosphere, dissolve in the same and change into a solution. Calcium chloride (CaCl2) is an example of a deliquescent substance.
• Efflorescence : Crystalline hydrated salts which on exposure to the atmosphere lose their water of crystallisation partly or completely and change into a powder. Sodium carbonate (Na2CO3.10H2O) is an efflorescent substance.
• Water of crystallization : It is the amount of water molecules which enter into lose chemical combination with one molecule of the substance on crystallisation from its aqueous solution.

Acids Bases and Salts Class 10 Important Questions given here are ready by our Experts teachers at icseboards as per the most recent ICSE Class 10 Syllabus and CISCE rules. These Acids Bases and Salts Class 10 Important Questions will assist students with saving a ton of time during their exams.
Acids Bases and Salts Class 10 Important Questions by icseboards are up to this point the best and most study material for ICSE Class 10 Chemistry.
Acids Bases and Salts Class 10 Important Questions will most likely increment your certainty and lessen the tension of assessment. Students can download the FREE PDF of Important Questions on Acids Bases and Salts Class 10 and use it to clear the entirety of their questions and inquiries and henceforth, excel in their exams.


