Students should refer to ICSE Class 10 Chemistry Question Paper solved Set G given below which will help them to prepare for the upcoming ICSE Chemistry exams. Students should read ICSE Chemistry Class 10 Books to make sure they are completely prepared and should also refer to ICSE Class 10 Chemistry Solutions to understand all questions and their answers.
ICSE Class 10 Chemistry Question Paper solved Set G
Answer to this paper must be written on the paper provided separately.
You will not be allowed to write during the first 15 minutes.
This time is to be spent in reading the Question Paper.
The time given at the head of this paper is the time allowed for writing the answers.
Section I is compulsory. Attempt any four questions from Section II.
The intended marks for questions or parts of questions are given in brackets [ ].
ICSE Class 10 Chemistry Question Paper solved Set G
SECTION – I (40 MARKS)
(Attempt all questions)
Question 1.
(a) Chosse from the following list of the substances, as to what matches the description from (I) to (v) given below :
[Acetylence gas, aqua fortis, coke, brass, barium chloride, bronze, platinum].
(i) An aqueous salt solution used for testing sulphate radical.
(ii) A catalyst used in the manufacture of nitric acid by Ostwald’s process.
(iii) A black powdery substance used for the reduction of zinc oxide during its extraction.
(iv) A gaseous hydrocarbon commonly used for welding purposes.
(v) The substance is an alloy of zinc, copper and tin. [5]
(b) What would you observe in each of the following cases?
(i) Ammonium hydroxide is first added in a small quantity and then in excess to a solution of copper sulphate.
(ii) Sugar crystals are added to a hard glass test tube containing concentrated sulphuric acid.
(iii) Copper is heated with concentrated nitric acid in a hard glass test tube.
(iv) Water is added to the product formed, when aluminium is burnt in a jar of nitrogen gas.
(v) When carbon monoxide is passed over heated copper oxide. [5]
(c) Give reasons as to why :
(i) the electrolysis of acidulated water is considered to be an example of catalysis.
(ii) almost 90% of all known compounds are organic in nature.
(iii) it is dangerous to burn methane in an insufficient supply of air.
(iv) hydrogen chloride can be termed as a polar covalent compound.
(v) the oxidising power of elements increases on moving from left to right along a period in the periodic table. [5]
(d) Fill in the blanks from the choices given below :
(i) In covalent compounds, the bond is formed due to the ……………… (sharing/transfer) of electrons.
(ii) Electrovalent compounds have a …………………(low/high) boiling point.
(iii) A molecule of ………………. contains a triple bond. (hydrogen, ammonia,nitrogen)
(iv) Across a period, the ionization potential …………………..(increases, decreases, remains same.)
(v) Down the group, electron affinity …………….. (increases, decreases, remains same).
(e) (i) Calculate the volume of 320 g of SO2 at stp. (Atomic mass : S = 32 and O = 16)
(ii) State Gay-Lussaic’s Law of combining volumes.
(iii) Calculate the volume of oxygen required for the complete combustion of 8.8g of propane (C3 H8). (Atomic mass : C = 14, O = 16, H = 1, Molar Volume = 22.4 dm3 at stp)
(f) Choose the correct answer from the options given below :
(i) This metal is a liquid at room temperature.
(a) Potassium
(b) Zinc
(c) Gold
(d) Mercury
(ii) Hydroxide of this metal is soluble in sodium hydroxide solution.
(a) Magnesium
(b) Lead
(c) Silver
(d) Copper
(iii) In the periodic table alkali metals are placed in the group …………..
(a) 1
(b) 11
(c) 17
(d) 18
(iv) Hydrogen chloride gas being highly soluble in water is dried by :
(a) Anhydrous calcium chloride
(b) Phosphorous penta oxide
(c) Quick lime
(d) Concentrated sulphuric acid.
(v) The brown ring test is used for detection of :
(a) CO2–3
(b) NO–3
(c) SO2–3
(d) Cl–
(vi) When dilute sulphuric acid reacts with iron sulphide, the gas evolved is ………………
(a) Hydrogen sulphide
(b) Sulphur dioxide
(c) Sulphur trioxide
(d) Vapour of sulphuric acid.
(vii) The functional group present in acetic acid is :

(b) Hydroxyl –OH
(c) Aldehydic –CHO
(d) Carboxyl –COOH
(viii) The unsaturated hydrocarbons undergo :
(a) a substitution reaction
(b) an oxidation reaction
(c) an addition reaction
(d) none of the above
(ix) The number of C – H bonds in ethane molecule are :
(a) Four
(b) Six
(c) Eight
(d) Ten
(x) Which of the following properties do not match with elements of the halogen family?
(a) They have seven electrons in their valence shell.
(b) They are highly reactive chemically.
(c) They are metallic in nature.
(d) They are diatomic in their molecular form.
(g) Write the balanced chemical equation for each of the following reactions :
(i) Sodium thiosulphate is reacted with dilute hydrochloric acid
(ii) Calcium bicarbonate reacts with dilute hydrochloric acid
(iii) Dilute sulphuric acid is poured over sodium sulphite
(iv) Lead nitrate solution is added to sodium chloride solution
(v) Zinc is heated with sodium hydroxide solution.
Answer.
(a) (i) Barium chloride
(ii) Platinum
(iii) Coke
(iv) Acetylene
(v) Bronze
(b) (i) On adding ammonium hydroxide in small amount, blue precipitates will appear. On adding ammonium hydroxide in excess, blue precipitates will dissolve forming deep blue solution.
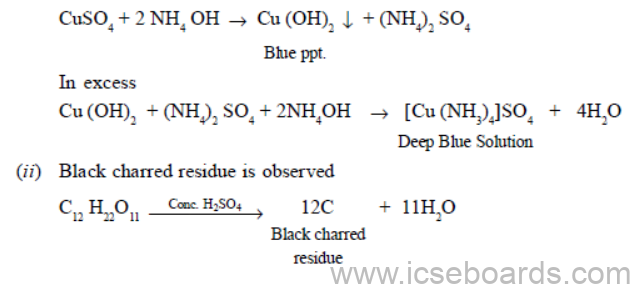

This is because carbon monoxide (CO) acts as a reducing agent and reduces copper oxide (CuO).
(c) (i) Because of two reasons :
1. It initiates the chemical reaction which increases the rate of reaction.
2. Secondly, same amount of acid is recovered at the end of reaction.
(ii) Because of ability of carbon to catenate i.e forms straight chain, branched chains or ring like compounds.
(iii) Because carbon monoxide is produced in an insufficient supply of air. This gas is extremely poisonous for human beings as it cuts off the oxygen supply by forming carboxyhaemoglobin in the blood.

Pure covalent bond exists between two elements which have similar electronegativities.
In hydrogen chloride, chlorine being more electronegative attracts the shared pair of electrons towards itself as a result hydrogen acquires partial positive charge and chlorine gets partial negative charge. Thus, hydrogen chloride can be termed as a polar covalent compound.
(v) Oxidising power means to accept electrons.
As we move from left to right along a periodic table, the size of element decrease, hold of nucleus increases, incoming electron is accepted easily thus oxidising power of element increases.
(d) (i) sharing
(ii) high
(iii) nitrogen
(iv) increases
(v) decreases
(e) (i) SO2
M.W. = 32 + 16 × 2 = 64
64 g of SO2 occupy 22.4 litres
1 g of SO2 will occupy 22.4 / 64 litres
320 g of SO2 will occupy 22.4 / 64 × 320 = 5. 6 × 20 = 112 litres
(ii) It states that “Whenever gases react they do so in volumes which bear a simple whole number ratio to one another and to the volumes of gaseous products, all volumes being measured under similar conditions of temperature and pressure.”
(iii) C3H8 + 5O2 → 3CO2 + 4 H2O
M.W. of C3 H8 = 12 × 3 + 8 = 44
Volume of 5O2 = 5 × 22.4 = 112 litres
44 g of propane requires = 112 litres of oxygen
1 g of propane requires = 112 /44 litres
8.8 g of propane requires = 112 / 44 × 8.8 =112 ×.2 = 22.4 litres

(g) (i) Na2 S2O3 + 2HCl → 2NaCl + SO2 + S↓ + H2O
(ii) Ca (HCO3)2 + 2HCl → CaCl2 + 2 H2O + 2 CO2 ↑
iii) Na2SO3 + H2SO4 (dilute) → Na2SO4 + H2O + SO2 ↑
iv) Pb (NO3)2 + 2NaCl → PbCl2 + 2NaNO3
(v) Zn + 2NaOH → Na2 ZnO2 + H2
ICSE Class 10 Chemistry Question Paper solved Set G
SECTION II (40 Marks)
(Attempt any Four Question )
Question 2.
(a) Differentiate between electrical conductivity of copper sulphate solution and copper metal. [3]
(b) Sodium hydroxide solution is added to the solutions containing the ions mentioned in List X. List Y gives the details of the precipitate. Match the ions with their coloured precipitates.


(c) During the electrolysis of copper (II) sulphate solution using platinum as cathode and carbon as anode :
(i) What do you observe as the cathode and at the anode? [1]
(ii) What change is noticed in the electrolyte? [1]
(iii) Write the reactions at the cathode and at the anode. [2]
Answer.

(b) (i) Pb2+ → White soluble in excess
(ii) Fe2+ → Dirty green
(iii) Zn2+ → White soluble in excess
(iv) Fe3+ → Reddish brown
(v) Cu2+ → Blue
(vi) Ca2+ → White insoluble in excess.
(c) (i) Cathode : Reddish brown deposition of copper occurs at cathode.
Anode : Colourless gas is evolved at anode.
(ii) On prolonged electrolysis, the blue electrolyte turns colourless.
(iii) Cathode : Cu2+ + 2e– → Cu
Anode : OH– — 1e– → OH
4OH → 2H2O + O2 ↑
Question 3.
(a) Answer the following questions :
(i) Name a metal which is found abundantly in the earth’s crust.
(ii) What is the difference between calcination and roasting?
(iii) Name the process used for the enrichment of sulphide ore.
(iv) Write the chemical formulae of one main ore of iron and aluminium.
(v) Write the constituents of electrolyte for the extraction of aluminium. [5]
(b) The diagram shows an experimental set up for the laboratory preparation of a pungent smelling gas. The gas is alkaline in nature.

(i) Name the gas collected in the jar.
(ii) Write the balanced equation for the above preparation.
(iii) How is the gas being collected?
(iv) Name the drying agent used.
(v) How will you find that the jar is full of gas?
Answer. (a) (i) Aluminium

(iii) Froth floatation process
(iv) Iron : Haematite → Fe2O3
Aluminium : Bauxite → Al2O3.2H2O
(v) Constituents of electroylte for the extraction of aluminium
Pure molten alumina → 20%
Cryolite → 60%
Fluorspar → 20%
(b) (i) Ammonia (NH3)
(ii) NH4 Cl + Ca (OH)2 → CaCl2 + 2H2O + 2NH3 ↑
iii) Downward displacement of air
(iv) Quick lime (CaO)
(v) Bring a rod dipped in HCl near it. Dense white fumes of ammonium chloride will be formed.
Question 4. (a) An organic compound with vapour density = 94 contains
C = 12.67%, H = 2.13%, and Br = 85.11%. Find the molecular formula. [Atomic mass : C = 12, H = 1, Br = 80] [3]
(b) Calculate the mass of :
(i) 1022 atoms of sulphur.
(ii) 0.1 mole of carbon dioxide. [Atomic mass : S = 32, C = 12 and O = 16 and Avogadro’s Number = 6 × 1023] [2]
(c) In the laboratory preparation of hydrochloric acid, HCl gas is dissolved in water.
(i) Draw a diagram to show the arrangement used for the absorption of HCl in water.
(ii) Why is such an arrangement necessary? Give two reasons.
(iii) Write the chemical equations for the laboratory preparation of HCl gas when the reactants are :
(a) below 200ºC
Answer.

E. F. = CH2 Br
Given V.D. + = 94
Molecular mass = 2 × V.D. = 2 × 94 = 188
M.F.M = (E.F.M)n
188 = (94)n
n = 188 /94 = 2
Molecular formula = (Empirical formula) × n = (CH2Br) × 2 = C2H4Br2
(b) (i) 6. 022 × 1023 atoms of sulphur weight = 32
1 atom of sulphur weigh = 32 / 6.022 1023
1022 atoms of sulphur weigh = 32 /6.022 1023 × 1022 = 0.531 g
(iii) 1 mole of CO2 weigh = 44 g
0.1 mole of CO2 weigh = 44 × 0.1 = 4.4 g
(c) (i)

Funnel arrangement used for absorption of HCl in water.
(ii) Two reasons for the use of funnel arrangement are : Prevents back suction of water into the flask.
Provides a large surface area for the absorption of the gas.

Question 5.
(a) Choose the correct word/phrase from within the brackets to complete the following sentences :
(i) The catalyst used for conversion of ethene to ethane is commonly……… . (nickel/iron/cobalt)
(ii) When acetaldehyde is oxidized with acidified potassium dichromate, it forms …….(ester/ethanol/acetic acid)
(iii) Ethanoic acid reacts with ethanol in presence of concentracted H2SO4, so as to form a compound and water. The chemical reaction which takes place is called…. . (dehydration/hydrogenation/esterification)
(iv) Write the equation for the reaction taking place between 1, 2 – dibromoethane and alcoholic potassium hydroxide.
(v) The product formed when ethene gas reacts with water in the presence of sulphuric acid is….. (ethanol/ethanal/ethanoic acid)
(b) Write balanced chemical equations for the following :
(i) Monochloro ethane is hydrolysed with aqueous KOH.
(ii) A mixture of soda lime and sodium acetate is heated.
(iii) Ethanol under high pressure and low temperature is treated with acidified potassium dichromate.
(iv) Water is added to calcium carbide.
(v) Ethanol reacts with sodium at room temperature.
Answer.
(a) (i) Nickel
(ii) Acetic acid
(iii) Esterification

Question 6.
(a) (i) With the help of equations, give an outline for the manufacture of sulphuric acid by the contact process.
(ii) What property of sulphuric acid is shown by the reaction of concentrate sulphuric acid when heated with
(a) Potassium nitrate
(b) Carbon? [5]
(b) (i) What is the special feature of the apparatus that is used in the laboratory preparation of nitric acid? [2]
(c) Write balanced chemical equations for the following :
(i) Chlorine reacts with excess of ammonia.
(ii) Ferric hydroxide reacts with nitric acid.
(iii) Zinc oxide dissolves in sodium hydroxide.
Answer.

(B) It behaves as an oxidising agent and oxidises carbon to carbon dioxide
C + 2H2 SO4 → CO2 + 2H2O + 2SO2 ↑
b) (i) All glass apparatus is used because the vapours of nitric acid are corrosive and destroy materials like rubber and cork.
(ii) The reaction mixture is not heated beyond 200 ºC because at higher temperature : The nitric acid would decompose :
4 HNO3 → 4NO2↑ + 2H2O + O2↑
The residue, sodium sulphate or potassium sulphate, forms a hard crust that sticks to the glass. Hence, its removal becomes difficult.
(c) (i) 8 NH3 (excess) + 3Cl2 → N2 +6NH4 Cl
(ii) Fe (OH)3 + 3HNO3 Fe (NO3)3 + 3H2O
(iii) ZnO + 2NaOH Na2 ZnO2 + H2O
Question 7. (a)
(i) Give the number of the group and the period, of the element having three shells with three electrons in valence shell.
(ii) By drawing an electron dot diagram, show the lone pair effect leading to the formation of ammonium ion from ammonia gas and hydrogen ion.
(iii) What happens to the crystals of washing soda when exposed to air? Name the phenomenon exhibited. [5]
(b) Name the method used for preparation of the following salts from the list given below:
(i) Sodium nitrate
(ii) Iron
(III) chloride
(iii) Lead chloride
(iv) Zinc sulphate
(v) Sodium hydrogen sulphate.
List :
(a) Simple displacement
(b) Neutralization
(c) Decomposition by acid
(d) Double decomposition
(e) Direct synthesis
Answer.


Ammonia has one lone pair of electrons which is donated to hydrogen atom forming a co-ordinate bond. The arrow represents a co-ordinate bond. The arrow points from the donor to the receptor atom.
(iii) It loses its water of crystallisation and becomes amorphous. This phenomenon is known as efflorescence.

(b) (i) B
(ii) E
(iii) D
(iv) A
(v) C