ICSE Class 10 students can refer to ICSE Class 10 Acids, Bases and Salts Notes given below which have been prepared as per the latest syllabus and guidelines issued by ICSE Board. All chapter wise revision notes for ICSE Class 10 Chemistry have been prepared by teachers have strong understanding of Chemistry. Read the notes prior to the exams to get better marks in exams
Acids, Bases and Salts ICSE Class 10 Chemistry Revision Notes
Students can refer to the quick revision notes prepared for Chapter Acids, Bases and Salts in Class 10 ICSE. These notes will be really helpful for the students giving the Chemistry exam in ICSE Class 10. Our teachers have prepared these concept notes based on the latest ICSE syllabus and ICSE books issued for the current academic year.
Revision Notes ICSE Class 10 Chemistry Acids, Bases and Salts
Please refer to the detailed notes below
Acid
An acid is a compound which when dissolved in water yields hydronium ions (H3O+) as the only positively
charged ions.

Classification of Acids
1. Depending on sources
Organic acid: Acids which are usually obtained from plants are called organic acids. They contain
carbon and hydrogen atoms.
Examples

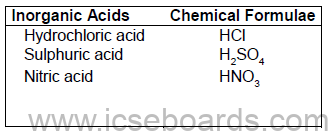
Inorganic (Mineral) acids: Acids which are obtained from minerals are known as inorganic acids.
Examples
2. Depending on strength
(a) Strength of an acid: The strength of an acid depends on the concentration of the hydronium ions
(H3O+) present in the aqueous solution of an acid.
i. Strong acids: A strong acid vigorously ionises in aqueous solution, thereby producing a high
concentration of hydronium ions (H3O+).
Examples: HNO3, HCl, H2SO4
ii. Weak acids: Weak acids ionise only partially in aqueous solution to produce ions and molecules.
Examples: H2CO3, CH3COOH, HCOOH
3. Depending on basicity
Basicity of an acid: The number of hydronium ions (H3O+) which can be produced by the ionisation of
one molecule of that acid in aqueous solution.
i. Monobasic acids: Acids which on ionisation in water

iii. Tribasic acids: Acids which on ionisation in water produce three hydronium ions (H3O+) per
molecule of the acid are known as tribasic acids.

4. Depending on concentration
The concentration of an acid means the amount of acid present in a definite amount of its aqueous
solution.
i. Concentrated acid: An acid which contains a very small amount of water or no water is called a
concentrated acid.
ii. Dilute acid: An acid which contains far more amount of water than its own mass is known as a
dilute acid.
5. Depending on molecular composition
i. Hydracids: Acids which contain hydrogen, a non-metallic element and no oxygen are called
hydracids. Examples: HCl, H2S, HBr, HI
ii. Oxyacid’s: Acids which contain oxygen, hydrogen and a non-metallic element are called
oxyacid’s. Examples: H2SO4, HNO3, H2CO3
Preparation of Acids
1. By synthesis
H2 + Cl2 → 2HCl
2. By the action of water on non-metallic or acidic oxides
SO3 + H2O → H2SO4
N2O5 + H2O → 2HNO3
3. By oxidation of non-metals
S + 6HNO3 → H2SO4 + 2H2O + 6NO2
P + H3PO4 → H3PO4 + H2O + 5O2
4. By displacement
NaCl + H2SO4 → NaHSO4+ HCl
NaNO3+ H2SO4 → NaHSO4+ HNO3
Properties of Acids
Physical properties
i. Sour in taste in aqueous solution.
ii. Turns blue litmus red.
iii. Some acids are solids and some are liquids at room temperature.
iv. All strong mineral acids have corrosive action on the skin and cause painful burns.
v. They are electrolytes, i.e. they conduct electricity in the aqueous state.
Chemical Properties
1. Reaction with active metals
Mg + 2HCl → MgCl2 + H2
2. Reaction with bases – Neutralisation
NaOH + H2SO4 → NaNO3 + H2O
3. Reaction with carbonates and bicarbonates
CaCO3 + 2HCl → CaCl2+ H2O + CO2
4. Reaction with sulphites and bisulphites
CaSO3 + 2HCl → CaCl2+ H2O + SO2
NaHSO3 + HCl → NaCl + H2O + SO2
5. Reaction with sulphides
ZnS + 2HCl → ZnCl2 + H2S
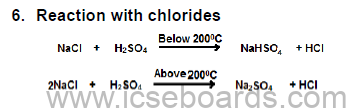
7. Reaction with nitrates
Pb (NO3)2 + 2HCl → PbCl2 + 2HNO3
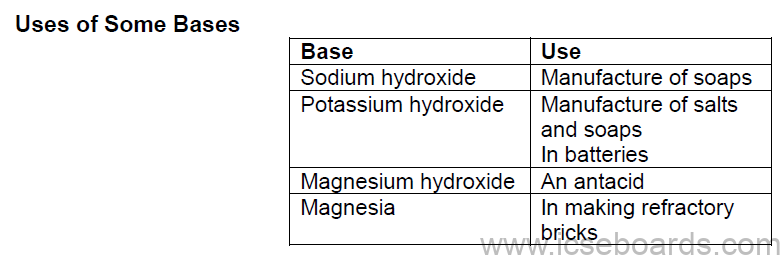
Uses of Some Acids

Bases
A base is either a metallic oxide or a metallic hydroxide or ammonium hydroxide which reacts with
hydronium ions of an acid to form salt and water only.
Basic Oxide
A basic oxide is a metallic oxide which contains the ion O2− and reacts with an acid to form salt and water.
Alkalis
An alkali is a basic hydroxide which when dissolved in water produces hydroxyl (OH−) ions as the only
negatively charged ions.

Classification of Bases
1. On the basis of strength
i. Strong base: It undergoes almost complete ionisation in aqueous solution to produce a high
concentration of OH− ions.

ii. Weak base: It undergoes only partial ionisation in aqueous solution to produce a low concentration
of OH− in solution.

2. On the basis of acidity
a. Acidity of a base: The number of hydroxyl ions (OH–) which can be produced per molecule of the
base in aqueous solution.
i. Monoacidic base: Bases which dissociate in aqueous solution to produce one hydroxyl ion
(OH–) per molecule of the base are called monoacidic bases.

ii. Diacidic base: Bases which dissociate in aqueous solution to produce two hydroxyl ions (OH–) per molecule of the base are called diacidic bases

iii. Triacidic base: Bases which dissociate in aqueous solution to produce three hydroxyl ions (OH–) per molecule of the base are called triacidic bases.

3: On the basis of composition
Concentrated alkali: It is an alkali with a relatively high percentage of alkali in its aqueous solution.
Dilute alkali: It is an alkali with a relatively low percentage of alkali in its aqueous solution.
Preparation of Bases
i. From Metals
2Mg + O2 → 2MgO
ii. By action of water or steam on reactive metals
2Na + 2H2O → 2NaOH + H2
iii. By the action of water on soluble metallic oxides
Na2O + H2O → 2NaOH
iv. By double decomposition
FeCl3 + 3NaOH→Fe (OH) 3 + 3NaCl
v. By the action of oxygen on metal sulphides
2ZnS + 3O2 → 2ZnO + 2SO2
vi. By decomposition of salts
CaCO3→ CaO + CO2
Properties of Bases
Physical properties
- 1. They have sharp and bitter taste.
2. They change red litmus blue.
3. Soapy substances, i.e. they are slippery to touch.
4. They are strong electrolytes.
5. They show mild corrosive action on the skin.
Chemical properties
Reaction with carbon dioxide
2NaOH + CO2 → Na2CO3 + H2O
Reaction with acids – Neutralisation
Ca (OH) 2 + 2HCl → CaCl2 + 2H2O
Reaction with metallic salts
CuSO4 + 2NH4OH → (NH4)2SO4 + Cu (OH) 2
pH Value
It represents the strength of acids and alkalis expressed in terms of hydrogen ion concentration.
pH of Solution
pH of a solution is the negative logarithm to the base 10 of the hydrogen ion concentration expressed in
mole per litre. pH = –log10 (H+)
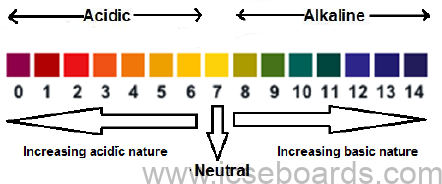
pH Scale
It is a scale showing the relative strength of acids and alkalis.
The normal pH scale ranges from 0 to 14 as shown below.
Indicators
They are complex substances which acquire separate colours in acidic and basic media.
Types of Indicators
a. Acid–base indicators: Common acid–base indicators such as litmus, methyl orange and
phenolphthalein can distinguish between acid and basic solutions, but they cannot determine the
strength of the solution.
b. Universal indicator: A universal indicator is a mixture of organic dyes which gives a definite colour
change over a wide range of pH.
Salts
A salt is a compound formed by the partial or total replacement of the ionisable hydrogen atoms of an acid
by a metallic ion or an ammonium ion.
Classification of Salts
1. Normal salts: The salts formed by the complete replacement of the replaceable hydrogen ion of an
acid molecule by a basic radical.
Example:
HCl + NaOH → NaCl + H2O
2. Acid salts: The salts formed by partial replacement of the replaceable hydrogen ion of an acid
molecule by a basic radical.
Example:
NaOH + H2SO4 → NaHSO4 + H2O
3. Basic salts: The salts formed by the partial replacement of the hydroxyl group of a di- or tri-acidic
base by an acidic radical.
Example:
Mg (OH) 2 + HCl → Mg (OH) Cl + H2O
4. Double salts: The salts formed by the union of two simple salts which dissolve in water and
crystallise.
Example:
Potash alum: K2SO4. Al2 (SO4)3. 24H2O
5. Mixed salts: Mixed salts are those salts which contain more than one basic or acidic radical.
Example:
Sodium potassium carbonate NaKCO3
6. Complex salts: Complex salts are those salts which on dissociation give one simple ion and one
complex ion.
Example:


Preparation of Insoluble Salts
1. By direct combination
Reaction: Pb + S → PbS
2. By combination of an acidic oxide with a basic oxide
Reaction: SO2 + CaO → CaSO3
3. Double decomposition
Reactions: BaCl2 + H2SO4 → BaSO4 + 2HCl
Laboratory Preparation of some Normal and Acid Salts
1. Iron (III) chloride or anhydrous ferric chloride
It is prepared by passing dry chlorine gas over heated iron.
Fe + Cl2 → FeCl3
2. Copper (II) sulphate
It is prepared by the reaction of copper oxide, copper hydroxides or copper carbonates with dilute
sulphuric acid.
CuO + H2SO4 → CuSO4 + H2O
Cu (OH)2 + H2SO4 → CuSO4 + 2H2O
CuCO3 + H2SO4 → CuSO4 + H2O
CuSO4 + 5H2O → CuSO4.5H2O
3. Zinc sulphate and iron (II) sulphate
It is prepared by the reaction of metals with dilute sulphuric acid.
Zn + H2SO4 → ZnSO4 + H2O
ZnSO4 + 7H2O → FeSO4.7H2O
4. Lead chloride
It is prepared by adding either dilute hydrochloric acid or sodium chloride solution to a solution of lead
nitrate.
Pb (NO3)2 + 2HCl → PbCl2 + 2HNO3
5. Calcium carbonate
It is prepared by adding sodium carbonate solution to a hot solution of calcium chloride.
CaCl2 + Na2CO3 → CaCO3 + 2NaCl
6. Sodium bicarbonate
It is prepared by passing excess of carbon dioxide gas through a saturated solution of sodium
carbonate.
Na2CO3 + CO2 + H2O → 2 NaHCO3
7. Neutralisation
It is the process by which H+ ions of an acid react completely with the [OH−] ions of a base to give salt
and water only.
Example: HCl (Acid) + NaOH (Base) → NaCl (Salt) + H2O (water)
Water of Crystallisation
It is the amount of water molecules which enter into loose chemical combination with one molecule of the
substance on crystallisation from its aqueous solution.
Hydrated Salt
The salts which contain a definite number of water molecules as water of crystallisation are called
hydrated salts.
Examples: Na2CO3.10H2O (washing soda), CuSO4.5H2O (blue vitriol)
Anhydrous Salt
A salt which does not contain any water of crystallisation is called an anhydrous salt.
Examples: NaCl, NaNO3, Pb(NO3)2
Deliquescence
Water soluble salts which on exposure to the atmosphere absorb moisture from the atmosphere, dissolve
in the same and change into a solution. The phenomenon is called deliquescence and the salts deliquescent.
Examples: CaCl2, MgCl2, ZnCl2
Efflorescence
Crystalline hydrated salts which on exposure to the atmosphere lose their water of crystallisation partly or
completely and change into a powder. This phenomenon is called efflorescent and the salts efflorescent.
Examples: CuSO4.5H2O, MgSO4.7H2O, Na2CO3.10H2O



