Students should refer to Previous Year Questions ICSE Class 10 Modern Physics with solutions that have been prepared by expert teachers of ICSE Class 10 Physics. These questions and solutions are based on past year papers that have come in examinations of ICSE Class 10. Students should understand the type of questions asked and the solutions provided. Also refer to ICSE Class 10 Physics Solutions
ICSE Class 10 Physics Modern Last Year Questions
Students should learn the important questions and answers given below for Chapter Modern in Physics for ICSE Class 10. These board questions are expected to come in the upcoming exams. Students of ICSE Class 10th should go through the board exams questions and answers for ICSE Class 10 Physics which will help them to get more marks in exams.
Board Exam Questions Modern ICSE Class 10 Physics
Previous Year Questions ICSE Class 10 Modern Physics
Short Answer Type Questions I
Question: State two characteristics of a good thermion emitter.
Answer: (i) Low work function
(ii) High melting point.
Question: State two factors upon which the rate of emission of thermions depends.
Answer: (i) Nature of metal surface
(ii) Temperature of surface
(iii) Surface area of the metal.
Question: When does the nucleus of an atom tend to be radioactive?
Answer: When the nucleus is unstable i.e., the neutron – proton ratio is out of the safety belt region.
n/p ratio is less than 1 or more than 1.5.
Question: Show by equations, the effect on the proton number Z and mass number A of the parent
nucleus brought about by the two types of radioactive decay.
Answer: Two types of radioactive decay are
(1) through the emission of a-ray
(2) through the emission of b-ray. Equation of emission of a-ray

Question: An element zSA decays to 85R222 after emitting 2 α particles and 1 β particle. Find the atomic number
and atomic mass of the element S.
Answer: A = 230
Z = 88
Question: A radioactive substance is oxidized. Will there be any change in the nature of its radioactivity? Give
a reason for your answer.
Answer: No change
As radioactivity is a nuclear phenomenon.
Question: (i) Why is a cathode ray tube evacuated to a low pressure?
(ii) What happens if the negative potential is changed on a grid?
Answer: (i) So that electrons may not loose energy due to collision with air molecules.
(ii) Number of electrons reaching the electrode & hence the current decreases
Question: (i) Name the high energetic invisible electromagnetic waves which help in the
study of the structure of crystals.
(ii) State an additional use of the waves mentioned in part (e) (i).
Answer: (i) γ–rays (Gamma Rays)
(ii) Treatment of cancer..
Question: (i) What is thermionic emission?
(ii) Name the unit in which the work function of a metal is expressed.
Answer: (i) The emission of electrons from a metal surface when heat energy is imparted to it, is
called thermionic emission.
(ii) eV (electron volt)
Q. 9. Which of the radioactive radiations occurs in the
following cases :
(i) Can cause severe genetical disorder?
(ii) Are deflected by an electric field?
Answer: (i) Gamma radiation can cause severe genetical disorder.
(ii) Since a, b are charged particles, so it gets deflected in the electric field.
Question: A radioactive nucleus undergoes a series of decays according to the sequence.

If the mass number and atomic number of X3 are 132 and 69 respectively, what is the mass number and atomic number of X?
Answer: According to the question,

Question: Fill in the blanks in the following sentence with appropriate words.
During the emission of b-particle, the……number remains the same.
Answer: MASS
Question: A mixture of radioactive substances gives off three types of radiations.
(i) Name the radiation which travels with the speed of light.
(ii) Name the radiation which has the highest ionizing power.
Answer: (i) g radiation.
(ii) a2He4 particle.
Question: The nucleus 84X202 emits an a-particle and forms the nucleus Y. Represent this change in the form
of an equation.
Answer: The equation for the given nuclear reaction is

Question: Complete the following nuclear changes

Answer:

Question: (i) Which radiation produces maximum biological damage?
(ii) What happens to the atomic number of an element, when the radiation named by you in part
(i) above are emitted?
Answer: (i) γ-radiation produces maximum biological damage.
(ii) There is no change in atomic and mass number of element formed after γ-radiation or emission.
Question: A certain radioactive nucleus emits a particle that leaves its mass unchanged but increases its atomic
number by one. Identity the particle and write its symbol.
Answer: As β-particle consists of –1β0 and after its emission,
atomic number increases by 1 and mass number
remains as it is. Its symbol is –01β0.
Question: How many a and b-particles are emitted when uranium nucleus 92U238 decays to lead 82Pb206?
Answer: As from the question, change in mass number is
238 – 206 = 32 and change in atomic number is
92 – 82 = 10.
As mass number is decreased by 32, so there is
emission of 8 a-particles (2He4).
Since the change in atomic number is only 10, so there must be 6 emission.
Question: Mention two possible sources of background radiations.
Answer: The two possible source as follows are :
(1) Earth, (2) Cosmic radiation.
Question: An element X changes to another element Y with the emission of β-particles. Write down the
equation showing changes in the nucleus. Take the proton number and mass number of X as Z and
A respectively.
Answer: The equation for the emission are as follows is :
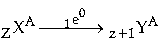
Question: Why do we prefer materials of low work function as thermionic cathode materials?
Answer: The use of such materials helps us to get much larger thermionic currents at much lower filament temperatures.
Question: State the relation between the eV and the joule.
Answer: 1 eV ≅ 1.6 × 10–19 J

Short Answer Type Questions II
Question: Answer the following questions based on a hot cathode ray tube.
(i) Name the charged particles.
(ii) State the approximate voltage used to heat the filament.
(iii) What will happen to the beam when it passes through the electric field?
Answer: (i) Electrons
(ii) 6V
(iii) It will deflect.
Question: State three factors on which the rate of emission of electrons from a metal surface depends.
Answer: (i) Nature of metal surface
(ii) Temperature of metal surface
(iii) Surface area of the metal.
Question: State one industrial use of a, b, and g radiation.
Answer: Uses of a-radiation They are used in smoke detectors. Uses of a-radiation Controls and measures
thickness of the material used in production of paper, plastic. Uses of g-radiation used in radiography
Question: (i) Define Work function of a metal.
(ii) State two factors on which the rate of emission
of thermions depend.
Answer: (i) Work function of a metal is defined as the minimum amount of energy required by an electron to just escape from the metal surface so as to overcome restraining forces.
(ii) The rate of emission of thermions (i.e., emitted electrons) depends on the following factors
(a) The nature of the metal surface.
(b) The surface area of metal.
Question: (i) Define Radioactivity.
(ii) A radioactive substance is oxidized. What change would you expect to take place in the nature of its radioactivity? Give a suitable reason.
Answer: (i) Radioactivity It is the process of spontaneous disintegration of the atomic nuclei with the emission of particles from within the nuclei of atoms.
(ii) If a radioactive substance is oxidized, then there will be no change that takes place in the nature of its radioactivity, because radioactivity is a natural phenomenon.
Question: Arrange a, b and γ rays in ascending order with respect to their
(i) Penetrating power.
(ii) Ionising power.
(iii) Biological effect.
Answer: (i) a < β < γ
(ii) γ < β < a
(iii) a < β < γ
Question: (i) In a cathode ray tube what is the function of anode?
(ii) State the energy conversion taking place in a cathode ray tube.
(iii) Write one use of cathode ray tube.
Answer: (i) Anode accelerates electrons and collimates into a fine beam.
(ii) Electric energy is converted into heat and then into light.
(iii) TV picture tube; check waveform of varying electric field (any one use).
Question: An atomic nucleus A is composed of 84 protons and 128 neutrons.
(i) The nucleus A emits an alpha particle and is transformed into nucleus B. What is the composition of nucleus B?
(ii) The nucleus B emits a beta particle and is transformed into a nucleus C. What is the composition of nucleus C?
(iii) Does the composition of nucleus C change if it emits gamma radiations?
Answer: (i) 82B208 or 82 protons and 126 neutrons
(ii) 83C208 or 83 protons and 125 neutrons
(iii) No.
Question: A nucleus 11Na24 emits a b-particle to change into magnesium (Mg).
(i) Write the symbolic equation for the process.
(ii) What are numbers 24 and 11 called?
(iii) What is the general name of 24
12Mg with respect to 24 11Na?
Answer: (i) The symbolic equation of this process is
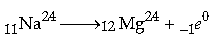
ii) 24 is the mass number and 11 is the atomic number.
(iii) 11Na24 and 12Mg 24 are isobars.
Question: (i) What is meant by radioactivity?
(ii) What is meant by nuclear waste?
(iii) Suggest one effective process for the safe disposal of nuclear waste.
Answer: (i) Radio activity is the spontaneous disintegration of an unstable nucleus to attain stability.
(ii) Nuclear waste are the material which remains after the use of radioactive element such as a, b
and γ particles in the form of radiation.
(iii) Nuclear waste must be put in a thick casks (dumbbell shaped structure), these are buried in the specially constructed deep underground stores.
Question: (i) Define radioactivity.
(ii) What happens inside the nucleus that cause the emission of b- particle?
(iii) Express the above change in the form of equation.
Answer: (i) Radioactivity is the spontaneous disintegration of an unstable nucleus to attain stability.
(ii) Inside the nucleus, to attain stability neutrons may change to a proton and emit b- particle.
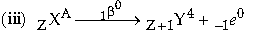
where, A = mass number and Z = atomic.
Question: (i) Name the radioactive radiation which have the least penetrating power.
(ii) Give one use of radio isotopes.
(iii) What is meant by background radiation?
Answer: (i) a-particle (2He4).
(ii) Radioactive isotopes are used in fuel for atomic energy reactor.
(iii) These are the invisible radioactive radiations such as a-b and γ produced during nuclear decay.
Question: (i) When does the nucleus of an atom become radioactive?
(ii) How is the radioactivity of an element affected, when it undergoes a chemical change to form a chemical compound?
(iii) Mention one use and one harmful effect of radioactivity.
Answer: (i) If the atomic number of an element is more than 82, then after it becomes radioactive. Atom number between 82 to 92 are good radioactive elements.
(ii) There is no change in the radioactivity during the chemical reaction because chemical reaction involves electrons of shell whereas radioactivity involves nucleons(i.e., neutron and protons)
(iii) Uses of radioactivity is in production electricity in nuclear reactor and in carbon dating by (C14) and in harmful effect it causes skin cancer and eye diseases.
Question: (i) Mention two important precautions that should be taken while handling radioactive materials :
(ii) State one use of radio–isotopes.
Answer: (i) Two important precautions of handling radioactive materials are as follows.
(a). Put them in thick lead box.
(b). Wear special protective clothes (lead coated) and gloves on the hand.
(ii) Radioactive isotopes are used in fuel for atomic energy reactor.
Question: (i) In a given hot cathode ray tube, what would be the effect of (a) a rise in the temperature of
the filament and (b) an increase in the anode voltage, on the particles forming the cathode ray beam.
(ii) Give one practical use of the cathode ray tube.
Answer: (i) (a) When a hotter filament is used, we have an increase in the number of particles (electrons) present in
the beam.
(b) when the anode voltage is increased, we have an increase in the energy of the beam particles.
(ii) The cathode ray tube is used as the picture tube of a television.
Question: Radioactive nuclei can decay in two different ways, Each of these brings about a change in the mass number A and the proton, number Z of the decaying parent nucleus. Write appropriate equation to show the effect of each type of decay on both A and Z.
Answer: Radioactive nuclei can decay either
(i). by emitting an alpha particle (4/2He) or (ii) by emitting a beta

(i) When a radioactive nucleus decays by emitting an alpha particle its mass number (A) decreases
by 4 units. While its proton number (Z) decreases by 2 units. The appropriate equation is

(iii) When a radioactive nucleus decays by emitting a beta particle, there is no change in its mass number (A) but its portion number increases

Long Answer Type Questions
Question: (i) What are free electrons?
(ii) Why do they not leave the metal surface on their own?
(iii) How can they be made to leave the metal surface?(State any two ways).
Answer: (i) The electrons which are not bound to the nucleus and free to move metal surface are called free electrons.
(ii) For electrons to have metal surface a minimum amount of energy is required. So they don’t leave the metal surface by their own.
(iii) By healing the surface by applying high electric field.
Question: It is observed that
(i) alpha particles and beta particles are deflected by an electric or magnetic field.
(ii) gamma rays are not deflected by either an electric or a magnetic field. Explain these observations.
Answer: (i) Alpha particles are nothing but helium nuclei.
They carry a positive charge (equivalent to 2 electronic charges). Therefore, they are deflected by an electric or a magnetic field in the sense in which a positively charged particle would be deflected by these fields. Beta particles are nothing but electrons. They carry a negative charge (equivalent to the charge of an electron). Therefore, they are deflected by an electric or a magnetic field in the sense in which a negatively charged particle would be deflected by these fields.
(ii) Gamma rays are nothing but high energy electromagnetic waves. They are electrically uncharged. Hence they are deflected by neither an electric nor a magnetic field.
Question: (i) Represent the change in the nucleus of radioactive element when a b particle is emitted.
(ii) What is the name given to element with same mass number and different atomic number?
(iii) Under which condition does the nucleus of an atom tend to be radioactive?
answer:
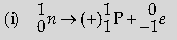
(ii) Isobars__________1
(iii) When
– Neutron proton ratio becomes more than 1.3 to 1.5
– Nucleus has more / excess mass,
– Nucleus has excess energy.
Question: Radioactive materials as an alternative source of energy must be used wisely. Give reasons to
justify this statement.
Answer: Radioactive materials as an alternative source of energy must be widely used, because these materials emit harmful radiations. The emission of harmful radiations cannot be controlled as the activity of radioactive materials is unaffected by any chemical or physical change, i.e., heating, freezing, applying strong electric or magnetic field or change of temperature or pressure. It is true that nuclear power plants are our major sources of energy but a large amount of radioactive materials and radiations escape to the atmosphere. Such materials exhibit vomiting, nausea, fever, loss of hair, leukaemia also killing the living tissues. That is why they are kept in thick lead blocks/ containers with a narrow opening so as to stop radiations coming out from other directions.
Question: A certain nucleus X has a mass number 14 and atomic number 6. The nucleus X changes to 7Y14
after the loss of a particle.
(i) Name the particles emitted.
(ii) Represent this change in the form of an equation.
(iii) A radioactive substance is oxidised. What change would you expect to take place in the nature of its radioactivity? Give a reason for your answer.
Answer: (i) Since during the -1b0 –mission there is gain in atomic number.

(iii) There is no change in the nature of radioactivity during oxidation. Because involvement of electrons takes place, whereas in radioactivity nucleons takes part in reaction.
Question: A nucleus zXA emit an alpha particle followed by γ-emission, therefore it emit two b-particles to form XB
(i) Copy and complete the value of A and Z for X3
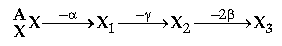
(ii) Out of a, b and γ radiation,
(a) Which radiation is the most perpetrating?
(b) Which radiation are negatively charged?
Answer:
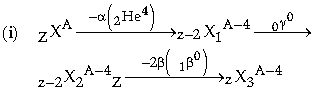
(ii) (a) The penetration power of γ is maximum
(b) As b is –1β0, so it is negatively charged particle in the radiation during radioactivity.
Question: (i) What happen to the atomic number of an element, when it emits
(a) An a-particle, (b) a b-paticle.
(ii) Explain why a and b-particles are deflected in an electric or a magnetic field but γ-rays are not deflected in such a field.
Answer: (i) (a) When an a-particles is emitted then atom number decrease by 2 and mass number decreases by 4.
(b) During b-emission, atomic number
increase by 1 as it consists of –1β0.
(ii) Since alpha particles is a helium nuclei (2He4)
and is positively charged particle and β particles
is (–1β4), negatively charged particle.
So in an electric field it experiences some force so, get deflected.
Question: The isotope of 92U238 decays by a emission to an isotope of thorium (Th). The thorium isotopes decays by b-emission to an isotope of protactinium (Pa). Write down the equation to represents these two nuclear charges.
Answer: The equation for a-emission is as follows

Question: Describe briefly, two properties of each of a and b particles.
Answer: Properties of a and b particles : Refer to Quick review page 137.
Question: (i) Name the particles given out during radioactive decays.
(ii) Show by equation, the effect on the proton number Z and mass number A of the parent nucleus brought about by the two types of radioactive decay.
(i) The particle given out in nuclear reaction are a-particles (2He4), b-particle (-1b0 ) and γ-particle (0γ0).
(ii) As the parent nucleus be X, so after a-decay is

Question: 12Mg24 αβ Al γ In the above nuclear reaction,
(i) 12Mg24 emit α, β-particle and is transformed to aluminium. What is the mass number and the atomic number of aluminium.
(ii) aluminium emit γ-rays. What is the resulting nucleus?
(iii) (a) which radiation or particle from radioactivity produces maximum biological damage?
(b) State three precautions that must be taken while handling a radioactive source.
Answer: The equation for radioactive reaction is

(i) The mass number will be 22 and atomic number will be 11.
(ii) When aluminium emit γ-rays there will be no effect in the nucleus formed.
(iii) (a) The radiation of γ-particles give most damage to the biological system.
(b) Three protective measures to handle radioactive material is as follows :
(1) Person should put on special lead lined aprons, lead gloves .
(2) Nucleus material must be kept in lead box.
(3) The workers must wear special film bags which can absorb nuclear radiations and they must have
compulsory medical check up.
Question: it is known that 238 / 92 U (uranium nucleus) decays to finally form the stable lead nucleus206 / 82 Pb. What is the number of alpha particles and beta particles emitted in this decay process?
Answer: The decay of 238 / 92 , from 206 / 82 Pb takes place via the
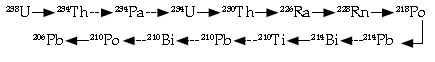
The solid arrows (8 in number) correspond to a-decay and the dotted arrows (6 in number) correspond to β-decay.
Therefore No. of a-particles emitted = 8
No. of b-particles emitted = 6
Alternatively,
Total change in mass number = 238 – 206 = 32.
It is only the emission of a particles that changes
the mass number (by 4 unit per emission) hence
number of a-particles emitted = 32 / 4 = 8. Also,
Total decrease in atomic number = 92 – β2 = 10 units. If only β a particles were emitted, the atomic number should decreases by 8 × 2 = 16 unit. A smaller (by 6 units) decrease in atomic number can come about only if 6 β-particles are also emitted. The emission of a negative b-particle increases the atomic number by 1 unit)
∴ No. of a-particles emitted = 8
No. of β-particles emitted = 6



