Students of ICSE Class 10 should refer to Electrolysis ICSE Class 10 Chemistry Questions below which have come in past board exams. You should always go through questions that have come in previous years. This will help you to understand the pattern of questions in ICSE Class 10 Chemistry and prepare accordingly. This will help you to get better marks in ICSE Class 10 Board Exams
ICSE Class 10 Chemistry Important Questions Electrolysis
Electrolysis is an important chapter in ICSE Class 10 Chemistry. Our faculty has prepared the following ICSE Class 10 Chemistry Questions and answers based on the latest syllabus and books issued for the current academic year. Please refer to the solved questions below and also see links provided for other chapters.
Electrolysis ICSE Class 10 Chemistry Questions
Electrolysis ICSE Class 10 Chemistry Questions
Indroduction
Terms involved in Electrolysis
Cell : A source of electricity is called a cell. The negative terminal of a cell is always drawn short and the positive terminal long.

Battery : A group of cells is called a battery.

Switch : A switch is used in the circuit to start or stop the flow of electricity.

Bulb : It tells us whether the current is flowing or not.
• Substances which allow electric current to flow through them are called conductors of electricity.
• Substances such as glass, rubber do not allow an electric current to flow through them are called insulators or non-conductors of electricity.
• The word electrolysis can be split into electro (meaning – electricity i.e. flow of electrons) and lysis (meaning – to break down).
1. Define the following with suitable examples or ionic equations.
(a) Electrolysis
(b) Electrolytes
(c) Strong electrolytes
(d) Weak electrolytes
(e) Electrolytic cell
(f) Electrodes
(g) Anode
(h) Cathode
(i) Anions
(j) Cation
(k) Electrolytic dissociation
(l) Ionisation
(m) Degree of dissociation of an electrolyte
(n) Metal activity series or electrochemical series
(o) Selective discharge of ions
(p) Electroplating
(q) Electrorefining
Ans. (a) Electrolysis : It is the decomposition of a chemical compound in the aqueous or fused state by the passage of a direct electric current resulting in the discharge of ions as neutral atoms at the respective electrodes.
Eg. Decomposition of NaCl electrolyte

(b) Electrolytes : These are the chemical compounds which conduct electricity either in the fused or in aqueous state.
e.g. dil. HCl and molten lead bromide.
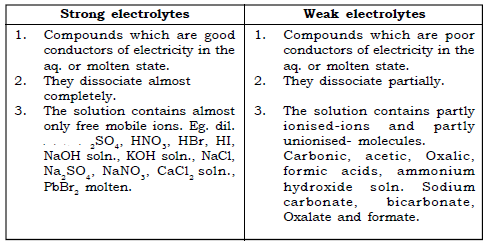
(c) Strong electrolytes : Compounds which are good conductors of electricity and dissociate almost completely in fused or in molten state.
e.g. dil. HCl, CuCl2 solution etc.
(d) Weak Electrolytes : Compounds which are poor conductors of electricity and dissociate partially in aqueous or in fused state.
e.g. Acetic acid, Sodium carbonate
(e) Electrolytic cell : A non conducting vessel containing the electrolyte in its aqueous or fused state for carrying out the process of electrolysis.
(f) Electrodes :- Two metal plates or wires or graphite rods or gas carbon rods immersed in the electrolyte through which the current enters and leaves the electrolytic cell are called electrodes.
(g) Anode : It is the electrode connected to the positive terminal of the battery where anions migrate and donate excess electron to get oxidised to neutral atoms.
(h) Cathode : It is the electrode connected to the negative terminal of the battery where cations migrate and gains excess electron to get reduced to neutral atoms.
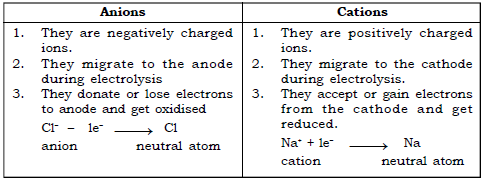
(i) Anion : They are negatively charged ions. They lose electrons to anode and get oxidised.

(j) Cation : These are positively charged ions. They gain electrons from the cathode and get reduced.

(k) Electrolytic dissociation : The process due to which an ionic compound in the fused (molten) state or in aqueous solution state dissociates into ions by passage of electric current through it is called electrolytic dissociation.
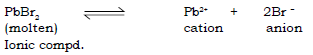
Ionisation may also involve formation of ions from atoms / molecules.

(m) Degree of dissociation of an electrolyte :- It is the extent to which an electrolyte (weak or strong) dissociates into its ions in the aqueous or molten state. Degree of dissociation is high for a strong electrolyte and low for a weak electrolyte.
(n) Metal activity series or electrochemical series :- Depending on the ease with which the metals lose their electrons and form ions. They are arranged in a series known as metal activity series or electrochemical series. The elements that ionize most readily are placed at the top of the series.
(o) Selective discharge of ions :- It is the preferential discharge of ions present in an electrolyte at the respective electrodes under particular conditions.
(p) Electroplating :- The electrolytic process of deposition of a superior metal on the surface of a base metal or article is called electroplating.
For e.g. : electroplating of an article of iron with Nickel.
(q) Electrorefining :- It is a process by which metals containing impurities are purified electrolytically to give a pure metal.
To summarize the process of electrolysis, we can say the following :
• Electrolytes dissociate to form negatively charged anions and positively charged cations.
• The ions conduct electricity through the electrolyte.
• Cations are attracted towards the negative electrode. They take the excess electrons from the electrode and neutralize themselves.
• Anions are attracted towards the positive electrode. They give up the excess electrons to the electrode and neutralize themselves. The electrolyte dissociates and the constituent elements of the salt are liberated at the electrodes.
2. What is electrolysis ?
Ans. It is the decomposition of a chemical compound (electrolyte) in the aqueous or fused (molten) state by the passage of a direct electric current resulting in discharge of ions as neutral atoms at the respective electrodes.
Decomposition of electrolyte :
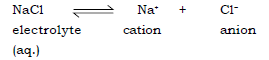
Discharge of ions as neutral atoms at the respective electrodes.
At cathode : Na+ + le– → Na (neutral atom)
Reduction reaction
At Anode : Cl– – le– → Cl (neutral atom)
Oxidation reaction
3. Distinguish between :-
(a) Electrolytes and Non-electrolytes.
Ans.

(b) Strong electrolytes and Weak electrolytes.
Ans.
(c) Anode and Cathode.
Ans.

(d) Anions and Cations.
Ans.
(e) Metal (eg Copper) and Electrolyte (eg aq. CuSO4)
Ans.

(f) Electrolytic dissociation and Ionisation.
Ans.

4. Give the main postulates of Arrhenius theory.
Ans. Arrhenius theory of electrolytic dissociation consists of following postulates :
• An electrolyte on dissolving in water dissociates into free ions and allows flow of electric current through it.
• The degree of dissociation is the extent to which an electrolyte dissociates into ions.
• Ions carry electric charge and conductivity of the solution depends upon the concentration of these ions.
• The solution is in the state of electrolytic equilibrium when the number of positive charges equals the number of negative charges.

5. Why ionic compounds are bad conductors of electricity in their solid state ? What happens to them when they are heated strongly?


Ans. Ionic compounds like NaCl are formed of positive (Na+) and negative (Cl–) ions, held together by strong electrostatic force of attraction which prevents the movement of ions. As free ions are not available in the solid state, they are bad conductor of electricity. When an ionic compound is heated strongly, the ions gain kinetic energy and start moving rapidly until the ions break lose and move freely. Thus the molten state of the compound conducts
electricity.
6. Why dry HCl gas does not conduct electricity, but when dissolved in water conducts ?
Ans. Hydrogen chloride is a polar covalent molecule, hence no ions are present in dry HCl gas. On adding hydrogen chloride to water due to its polar nature, ionisation of HCl occurs to produce H+ and Cl– ions, which are capable of conducting electricity. Hence HCl in water conducts electricity.

7. Give an experiment to show whether a given compound (acid, base or salt) in the fused or aqueous state is a) A strong electrolyte b) A weak electrolyte.
Ans. Aim : To determine whether a given compound is in the fused or aq. state is a) A strong electrolyte b) A weak electrolyte
Apparatus : A voltameter as shown in diagram.
Electrolyte : Electrolytic solution of the compound.
Electrodes : Graphite electrodes connected to a bulb and a current source.
Procedure : The given compound (acid, base, salt) in the fused or aqueous state is placed in the electrolytic cell and the current is switched on.
Observations : The conduction of electricity through the electrolyte is indicated by the glow of the bulb.
a. If the given compound i.e. acid, base (alkali) or salt solution is a strong electrolyte, the bulb glows brightly, when the current is switched on.
b. If the given compound is a weak electrolyte, the glow of the bulb is very dim, when the current is switched on.
c. If the given compound is a non – electrolyte, the bulb does not glow, when the current is switched on.

8. Why is electrolysis considered as a redox process?
Ans. In electrolysis, the cation moves towards cathode and is reduced to corresponding metal by gaining electron; where as the anion moves towards the anode and is oxidised to the corresponding non-metal by losing electrons, Since both oxidation and reduction take place during electrolysis, it is considered as a redox reaction.
9. Discuss the discharge of ions at electrodes depending on their position in the electrochemical series.
Ans.

• Higher the position of metal in the series greater is the reactivity and capacity of formation of positive ions.
• Cations at the top of series are discharged at cathode with greatest difficulty.
• Lower the position of metal in the series lesser is the reactivity and capacity of formation of positive ions.
• Cations at the lower end of series are discharged at cathode most readily.
10. What is selective discharge of ions ? State the factors influencing it?
Ans. The preferential discharge of ions present in an electrolyte at the respective electrodes under particular conditions is known as selective discharge of ions.
Factors affecting selective discharge :
i. Relative position of the cation or anion in electrochemical series : Lower the position of the ion, greater the tendency to be liberated at the respective electrode.
ii. Concentration of the ions in the electrolyte : Higher the concentration of the ion, greater is the probability of it being discharged at the respective electrodes.
iii. Nature of the electrode : Platinum or graphite electrodes are inert and do not take part in electrolysis, while metallic electrodes eg. Copper, nickel, silver are active and take part in electrolysis.
11. When a dilute aqueous solution of sodium chloride is electrolysed between platinum electrodes, hydrogen gas is evolved at the cathode but metallic sodium is not deposited. Why?
Ans. Position of hydrogen is much below that of sodium in the electrochemical series. So, hydrogen is preferentially discharged at the cathode.
12. During electrolysis of copper sulphate solution using platinum electrode oxygen gas is liberated, but on using copper electrode oxygen gas is not liberated. Why?
Ans. Platinum or graphite electrodes are inert and do not take part in electrolysis while metallic electrodes eg. Copper, nickel, silver are active and take part in electrolysis. For eg. Electrolysis of CuSO4(aq).

Nature of electrode determines the preferential ion discharged.

If electrode is an active electrode [Cu] the anions [SO4]-2 and OH– migrate to anode but are not discharged instead the active electrode itself loses electrons and form ions.
Electrolysis of some important compounds
13. Answer the following questions with respect to electrolysis of fused or molten lead bromide.

(a) Name the products formed at the cathode and the anode.
Ans. In the electrolysis of molten lead bromide, the products are:
At the cathode : Lead
At the anode: Vapours of Bromine
(b) It was noticed that the bulb lits up (i.e. the current started to flow) only when lead bromide is melted and not before. Explain why this is so.
Ans. The bulb lits up because molten lead bromide is an electrolyte. In the solid state, lead bromide is a non-conductor as Pb2+ and Br- ions are tightly held together by strong electrostatic forces of attraction, and they are not free to move towards their respective electrodes. However, on melting, the ions become free and can migrate towards the electrodes during electrolysis. Hence, it conducts electricity.
(c) What is the material used for the electrode and why is it preferred over other electrode?
Ans. The electrodes are made up of graphite. Graphite electrode is prefered to other inert electrode, since it is unaffected by the reactive bromine vapours.
(d) Why should the crucible be heated continuously throughtout the electrolysis?
Ans. In solid state lead bromide is a non-conductor of electricity, whereas it conducts electricity in molten state. The melting point of lead bromide is 308oC, hence the crucible has to be heated to maintain lead bromide in molten state.
(e) Why is the crucible made of silica?
Ans. The crucible is made of silica since it is non-reactive, withstands high temperature and is almost a non-conductor of electricity.
(f) Give the electrode reactions
Ans. At Cathode :
Pb2 + 2e– → Pb
At anode :
2Br1- – 2e– → 2 Br
Br + Br → Br2
(g) What do you observe at cathode and anode during electrolysis of molten or fused lead bromide.
Ans. At cathode : Silvery grey coloured metal is deposited.
At anode : Reddish brown vapours are seen.
14. Answer the following with respect to electrolysis of acidified water.

(a) Why is electrolysis carried out on acidified water.
Ans. Pure water is almost a non-electrolyte and does not conduct electricity. On addition of traces of dilute sulphuric acid, water ionises to H+ and OH– ions, since it is a polar covalent compound and thereby conducts electricity.
(b) Out of nitric acid and sulphuric acid which acid is preferred and why?
Ans. Sulphuric acid is preferred for acidification since nitric acid is volatile and may decompose and also the nitrate radical formed in the solution may tend to interfere with the electrolytic reaction.
(c) Name the ions present in the solution. Give the electrolytic reaction taking place in the solution.
Ans. H+, OH– and SO42– ions are present in the solution.
Electrolytic reaction :

(d) Name the main product of discharge of anion at the anode and write the anode reaction.
Ans. Oxygen gas is liberated at the anode
OH– – 1e– → OH × 4
4OH → 2H2O + O2
(e) Name the main product at the cathode and write the reaction.
Ans. Hydrogen gas is liberated at the cathode.
H+ + 1e– → H × 4
2H + 2H → 2H2
(f) What is the nature of electrodes? Why is it preferred?
Ans. Inert platinum electrodes are used. It is preferred over graphite electrode because it is non reactive with the gases liberated.
(g) During electrolysis of acidified water, the concentration of sulphuric acid at the cathode decreases.
Ans. The H+ ions are discharged at the cathode whereas OH– and SO4–2 ions move towards the anode but only OH– ions are discharged at anode. Hence concentration of SO4–2 ions increases at anode, where they combine with H+ ions forming sulphuric acid. Hence, concentration of sulphuric acid at anode increases and at cathode decreases but total concentration remains same.
(h) The ratio of hydrogen and oxygen liberated at the cathode and anode is in the ratio 2 : 1 by volume.
Ans. As per the electrolytic reactions 4H+ ions are needed at the cathode and 4OH– ions at the anode and two molecules of water are produced at the anode.
Hence for every two molecules of water, two molecules of hydrogen and one molecule of oxygen are liberated at cathode and anode respectively.

(i) Electrolysis of acidified water is considered as an example of catalysis.
Ans. Pure water is almost a non-electrolyte and will not normally conduct electricity. It consists almost entirely of molecules. It can be electrolytically decomposed by addition of traces of dilute sulphuric acid which dissociate as hydrogen [H1+] and sulphate [SO42–] ions and help in dissociating water into [H1+] and [OH1–] ions, water being a polar solvent. Electrolysis of acidulated water is hence considered to be an example of catalysis.
15. Answer the following with respect to electrolysis of aq. copper sulphate using cathode copper and platinum anode.
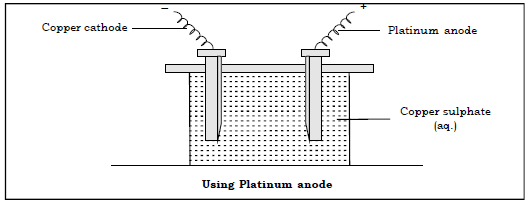
(a) Name the ions present in the electrolyte.
Ans. Cu2+, H+, OH–, SO42–
(b) Name the ions migrating towards anode and the product discharged.
Ans. SO42– and OH–
Oxygen gas is discharged at anode.
(c) Name the ions migrating towards cathode and the product obtained.
Ans. H+ and Cu2+ are the ions migrating towards cathode.
Copper metal is deposited at the cathode.
(d) What is observed at cathode and anode.
Ans. A brownish coloured metal is deposited at the cathode
A colourless gas is liberated at anode which rekindles the glowing wooden splinter.
(e) The blue colour fades when platinum electrode are used. Explain.
Ans. If platinum anode is used the blue colour of CuSO4 solution fades since the blue Cu2+ ions which are discharged at the cathode are not replaced or added at the anode.
(f) Give the electrode reaction.
Ans. At cathode
Cu2+ + 2e– → Cu
At anode
OH– –1e– → OH × 4
2OH → H2O + [O] × 2
4OH → 2H2O + O2
[O] + [O] → O2
(g) Name the spectator ions in the solution.
Ans. H+ and SO42– are the spectator ions in the solution.
16. Answer the following with respect to electrolysis of aqueous copper sulphate solution using copper electrodes.

a) Give the electrolytic reaction in the solution
Ans.

(b) Give the electrode reactions
Ans. At cathode :
Cu2+ + 2e– → Cu
At anode :
Cu – 2e– → Cu2+
(c) State your observation at cathode and anode.
Ans. A brownish coloured metal is deposited at the cathode.
Anode diminishes in size.
(d) The blue colour of aq. copper sulphate solution remains unchanged during electrolysis using copper electrodes explain.
Ans. For every Cu2+ ion discharged at the cathode as neutral copper atoms (Cu), a copper ion (Cu2+) is released to the solution at the anode and hence the total number of Cu2+ ions remains the same. Therefore the blue colour of the electrolyte does not fade.
17. State the reason for difference in product formed at the anode during electrolysis of aqueous CuSO4 solution using
(i) Active -Electrode – copper anode.
(ii) Inert electrode – platinum anode.
Ans. (i) At anode, no product is formed. During electrolysis of aqueous CuSO4 solution, SO4-2 and OH– ions migrating towards anode are not discharged, instead copper from anode enters the solution as Cu2+ ions.As copper anode loses electrons more easily.
(ii) At anode oxygen is formed as product. SO4-2 and OH– ions migrate to the anode but OH– ions are discharged. Since it is lower in the electrochemical series than SO42–.
Application of Electrolysis
18. Give the important application of electrolysis.
Ans. The main applications of electrolysis are :
(a) Electroplating of metals
(b) Electro – refining or purification of metals
(c) Extraction of metal
Electroplating of metals
19. What is electroplating?
Ans. The electrolytic process of deposition of a superior metal (eg. nickel, silver, chromium, gold) on the surface of a base metal or article (eg. copper, iron, brass) is called electroplating.
20. Why should an article be electroplated?
Ans. (i) Electroplating prevents corrosion or rusting of an article.
(ii) It makes the article attractive and gives it an expensive appearance.
21. Give reasons :
(a) The article to be electroplated is always placed at the cathode.
Ans. During electrolytic reaction the metal is always deposited at the cathode by gain of electrons. Since the electroplating is to be done on the surface of article, it is connected to the cathode.
(b) The metal to be plated on the article is always made the anode and has to be replaced periodically.
Ans. The metal anode is the superior metal which is to be electroplated on the article. Anode continuously dissolves as ions in the solution which migrates towards cathode and diminishes in mass and is hence replaced periodically.
(c) Electrolyte must contain, ions of the metal used for plating on the article.
Ans. The electrolyte is the substance which dissociates into ions of the metal which migrate towards the cathode and are deposited as neutral metallic atoms on the cathode (article).
(d) During electroplating a low current and for a longer time should be used.
Ans. Higher current causes uneven deposition of the metal. Longer time and low current initiates a smooth, firm and long lasting deposition.
(e) During electroplating a direct current and not A.C. current should be used.
Ans. A.C. current causes discharge and ionisation to alternate at the cathode thus giving no effective coating.
22. Answer the following questions with respect to electroplating of an article with nickel.
(a) Name the electrolyte used.
Ans. Nickel sulphate solution.
(b) At which electrode is the article to be plated kept?
Ans. At cathode.
(c) At which electrode is the nickel plate placed.
Ans. At anode.
(d) Give the equation for the electrolytic dissociation occuring in the solution.
Ans.

(e) Name the ions present in the solution.
Ans. Ni2+, H+, SO42–, OH–
(f) Name the ions migrating towards the anode. Which ion would get discharged and why?
Ans. SO42– and OH– migrate towards the anode but neither are discharged. Nickel being an active electrode itself decomposes to give Ni2+ ions into the solution.
(g) Give the electrode reaction.
Ans. At cathode
Ni2+ + 2e– → Ni
At anode
Ni – 2e– → Ni2+
23. Answer the following questions on the basis of electroplating spoon with silver.

(a) Name the electrolyte.
Ans. Sodium argentocyanide or sodium silver cyanide (Na[Ag(CN)2])
(b) Why is the above electrolyte preferred?
OR
Why is silver nitrate not used as the electrolyte?
Ans. Sodium silver cyanide is a complex salt, hence the migration of ions is slow as compared to that from silver nitrate. Hence an even deposition of the metal silver is obtained on the article. Therefore sodium argentocyanide is preferred to silver nitrate.
(c) What ions are be present in the electrolyte?
Ans. Na+, Ag+, CN-, H+ and OH– ions.
(d) What should be the nature of the anode and cathode?
Ans. The anode should be made up of pure block of silver. The cathode should be made up of the article to be electroplated (spoon).
(e) Give the equation of the reactions at the electrode.
Ans. At cathode

24. What is electro – refining of metals ?
Ans. It is the process by which metals containing impurities are purified electrolytically to give a pure metal.
25. How do we get pure copper metal from impure copper by using copper sulphate as electrolyte. Explain.
Ans. Electro – refining of copper
i. Electrolyte : Aqueous copper sulphate solution (acidified)
ii. Nature of electrode :
Cathode : Pure thin sheet of copper
Anode : Impure block of copper.
iii. Electrode Reaction :

iv. Reaction at cathode :
Cu2+ ions are discharged at the cathode as neutral copper atoms.
Cu2+ + 2e–→ Cu [Thus pure copper is getting deposited at pure thin copper placed as cathode]
v. Reaction at anode :
SO4 2– and OH1– ions migrate to the anode but neither are discharged.
Copper anode loses electrons to give Cu2+ ions in solution.
Cu – 2e– → Cu2+ (cation)
Hence anode diminishes in mass.
26. What is electrometallurgy?
Ans. Extraction of metals by electrolysis is called electrometallurgy. Metals comparitively higher in the electrochemical series are extracted by electrolysis.
27. How are following metals extracted.
a. Potassium b. Sodium c. Calcium d. Aluminium
Ans. Metals like potassium, sodium, calcium and aluminium are extracted by electrolysis of their fused salts. Their oxides are highly stable and do not decompose on thermal decomposition.
a. Extraction of Potassium :
Electrolyte : Potassium bromide (fused)
Electrode : Cathode : Iron
Anode : Graphite
Electrolytic Reaction : Potassium bromide (fused)
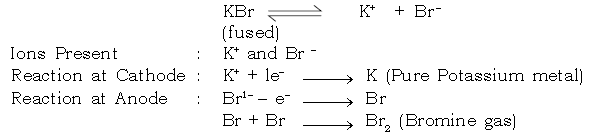
b. Extraction of Sodium :
Electrolyte : Sodium chloride (fused)
Electrode : Cathode : Iron
Anode : Graphite
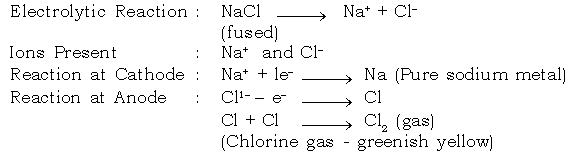
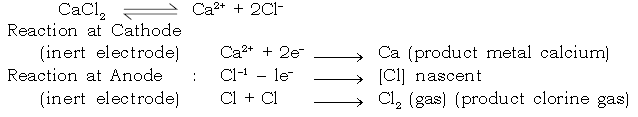
c. Extraction of Calcium :
Electrolyte : Calcium chloride [Fused]
Electrode : Cathode : Iron
Anode : Graphite

d. Extraction of Aluminium :
Electrolyte : Pure alumina (Al2O3) with cryolite (Na3AlF6)
and fluorspar [CaF2]
Electrode : Cathode : Gas carbon lining
Anode : Thick carbon rods

ADDITIONAL QUESTIONS
1. Name the following :
(1) Compounds which conduct electricity when dissolved in water or in the molten state.
Ans. Electrolytes.
(2) The decomposition of a chemical compound in the aqueous or fused state by the passage of direct
electric current.
Ans. Electrolysis.
(3) Electrode connected to the positive terminal of the battery.
Ans. Anode.
(4) Electrode connected to the negative terminal of the battery.
Ans. Cathode.
(5) Compound which does not conduct electricity in aqueous or molten state.
Ans. Non – electrolytes.
(6) Electrolytes which almost completely dissociate in fused or aqueous solution. state.
Ans. Strong electrolyte.
(7) Electrolytes which are partially dissociated in fused or aqueous state.
Ans. Weak electrolytes.
(8) It is the vessel in which electrolysis is carried out.
Ans. Electrolytic cell OR Voltameter.
(9) Ions which migrate to anode.
Ans. Anions.
(10) Ions which migrate to cathode.
Ans. Cations.
(11) The number of positive charges equals the number of negative charges in the electrolytic solution.
Ans. Electrolytic equilibrium.
(12) The process due to which an ionic compound in the fused or in aqueous state dissociates into ions by passage of electric current.
Ans. Electrolytic dissociation.
(13) An ionic compound, when added to water, it dissociates.
Ans. NaCl.
(14) A polar solvent.
Ans. Water.
(15) A metal which ionizes most readily.
Ans. potassium/ calcium/ sodium.
(16) Metal which ionizes least readily.
Ans. Silver/ mercury.
(17) It is the preferential discharge of ions present in an electrolyte at respective electrodes.
Ans. Selective discharge of ions.
(18) An inert electrode.
Ans. Iron/ graphite/ platinum.
(19) An active electrode.
Ans. Copper/ nickel/ silver.
(20) Product formed at anode during electrolysis of molten lead bromide.
Ans. Bromine vapours.
(21) Acid used for the acidification of pure water during electrolysis.
Ans. Dilute sulphuric acid.
(22) Product formed at cathode during electrolysis of acidified water.
Ans. Hydrogen gas.
(23) Product at anode during electrolysis of acidified water.
Ans. Oxygen gas.
(24) Product at anode during electrolysis of aqueous copper sulphate with inert electrode.
Ans. Oxygen gas.
(25) Product at anode during electrolysis of aqueous copper sulphate using active copper electrodes.
Ans. Copper ions.
(26) The electrolytic process of deposition of superior metal on the surface of baser metal.
Ans. Electroplating.
(27) The electrode at which the article to be plated is placed.
Ans. Cathode.
(28) The electrode at which the metal to be plated is placed.
Ans. Anode.
(29) Anode used during electroplating of an article with nickel.
Ans. Plate or block of nickel.
(30) The electrode which diminishes in mass during electroplating.
Ans. Anode.
(31) The process by which metals containing impurities are purified electrolytically to give a pure metal.
Ans. Electro refining.
(32) Metals generally refined by electrolysis.
Ans. Zinc, lead, copper, mercury, silver.
(33) The process of extraction of the metals by electrolysis.
Ans. Electro metallurgy.
(34) Metals which are generally extracted by electrolysis.
Ans. Potassium, sodium, calcium, magnesium, aluminium.
(35) The electrolytic cell during electrolysis of acidified water.
Ans. Hoffman’s voltameter.
(36) Metal that can be extracted from their oxides by thermal decomposition.
Ans. Mercury and silver.
(37) A salt which is a weak electrolyte.
Ans. Sodium carbonate or sodium bicarbonate.
(38) A base which is a weak electrolyte.
Ans. Ammonium hydroxide or calcium hydroxide
(39) A positively charged non-metallic ion.
Ans. Hydrogen ion (H+)
(40) The electrode at which reduction occurs.
Ans. Cathode
(41) A non-metallic element which is a conductor of electricity.
Ans. Graphite
(42) The electrode at which oxidation occurs.
Ans. Anode
(43) Name a liquid which is a non – electrolyte.
Ans. Sugar solution.
(44) Name a salt which in the fused state is a strong electrolyte.
Ans. Molten lead bromide.
2. Name the product at cathode and anode during electrolysis of :
a. Molten lead Bromide with inert electrode.
Cathode : lead metal, Anode : Bromine vapours
b. Molten Sodium chloride with inert electrodes.
Cathode : Sodium metal, Anode : Chlorine gas
c. Acidified copper sulphate solution with inert electrodes.
Cathode : Copper metal Anode : Oxygen gas
d. Acidified water with inert electrodes.
Cathode : Hydrogen gas Anode : Oxygen gas
e . Dilute Hydrochloric acid with inert electrodes.
Cathode : Hydrogen gas Anode : Oxygen gas
f. Concentrated hydrochloric acid with inert electrodes.
Cathode : Hydrogen gas Anode : chlorine gas
3.(a) Name the metals extracted by electrolysis.
Ans. Sodium, Potassium, Calcium, Magnesium etc.
(b) Name the compounds from which these metals are extracted.
Ans. These metals are extracted from their chloride i.e.
Sodium from Sodium chloride
Potassium from Potassium chloride
Calcium from Calcium chloride
Magnesium from Magnesium chloride
(c) What is the state of compounds from which these metals are extracted?
Ans. They are extracted from their fused or molten state.
4. Name all the particles present in.
(a) Sodium chloride solution.
Ans. Sodium ions, chloride ions, hydrogen ions, hydroxyl ions and water molecules.
(b) Molten sodium chloride.
Ans. Sodium ions, chloride ions.
(c) Sulphurous acid.
Ans. Hydrogen ion, sulphate ion, hydroxyl ions, sulphurous acid molecule, water molecule.
(d) Carbon tetrachloride.
Ans. Molecules of carbon tetrachloride.
5. Name the solution which contain.
(a) Only ions or mostly ions.
Ans. Any strong electrolyte e.g. dil. sulphuric acid.
(b) Only molecules.
Ans. Any non – electrolyte e.g. distilled water.
(c) Ions as well as molecules.
Ans. Any weak electrolyte e.g. carbonic acid.
6. State which of the following solutions contain (i) molecules only (ii) ions only (iii) both molecules and ions.
CS2, CH3COOH, NH4OH, NaOH, dil HNO3, Na2CO3, CuCl2 aq. Oxalic acid, Pure H2O, Kerosene.
Ans. Molecules only : CS2, Pure H2O, Kerosene
Ions only : NaOH soln, dil HNO3, CuCl2 aq.
Molecules and ions : CH3COOH, NH4OH, Na2CO3, Oxalic acid
7. Select the correct word from the words in bracket to complete the sentence.
(i) The electrode at which anions donate excess electrons and are oxidised to neutral atoms is the anode. (anode/ cathode)
(ii) On electrolysis, Ag1+ and H1+ ions migrate to the cathode (cathode/ anode) and Ag1+ (Ag1+ / H+1) are discharged.
(iii) Electrolysis is a/an redox (oxidation, reduction/ redox) reaction in which reduction reaction takes place at cathode. (anode/cathode)
(iv) According to Arrhenius theory the amount of electricity conducted by the electrolyte depends on concentration (nature/concentration) of the ions in the solution.
(v) Salts ionize in aq. solution, on passage of electric current to give positive (negative/ positive) ions other than
H+ ions.
(vi) Pure water consists of almost molecules (ions/molecules). We can expect that pure water will not (will/will not) normally conduct electricity.
(vii) With platinum electrodes hydrogen is liberated at cathode (Cathode/ anode) and oxygen at the anode (anode/cathode) during the electrolysis of acidified water.
(viii)Powdered sodium chloride does not conduct an electric current, but it does so in molten. (molten/gaseous) state.
(ix) Substances which conduct electricity in solid state are generally metals.(electrolytes/metals)
(x) The electron releasing tendency of zinc is more (more/less) than that of copper.
8. Choosing only words from the following list, write down the appropriate words to fill in the blanks below : anions, anode, cathode, cations, electrode, electrolyte, nickel, voltameter.
Ans. To electroplate an article with Nickel requires an :
(a) Electrolyte which must be a solution containing
(b) nickel ions. The article to be plated is placed as the
(c) cathode of the cell in which the plating is carried out The
(d) anode of the cell is made from pure nickel. The ions which are attracted to the negative electrode and discharged are called
(e) cations.
9. If the compound formed between X (a metal with a valency 2) and Y (a nonmetal with a valency 3) is fused and an electric current passed through the molten compound, element X will be obtained at the cathode and Y at
the anode of the electrolytic cell.
10. Select the correct answer from the list in bracket.
(i) The cation discharged at the cathode most readily (Fe2+, Cu2+, Pb2+, H1+) Cu2+
(ii) The anion discharged at the anode with most difficulty (so42-, Br1-, NO1-,3, OH1-) SO4-2
(iii) The metallic electrode which does not take part in an electrolytic reaction. (Cu, Ag, Fe, Ni) Fe
(iv) A covalent compound which in aqueous state conducts electricity (CH4,CS2, NH3, C2H4) NH3
11. Match the statement 1 to 5 with their answers, selected from A to J.
A : Cathode, B : Sucrose soln. C : Cl1–, D : formic acid,
E : Electro metallurgy, F : Ammonia, G : Mg2+, H : electro refining,
I : Sulphur dioxide, J : Anode
Ans. 1. A compound containing molecules only.
Sucrose solution
2. A compound which ionizes in solution state but not in gaseous state.
Ammonia
3. The ion which accepts electrons from the cathode and gets reduced to neutral atom.
Mg2+
4. The electrode to which the cyanide ions of aq. Na[Ag(CN)2] migrate during electrolysis.
Anode
5. An application of electrolysis in which anode does not generally diminish in size.
Electrometallurgy
12. (a) Arrange the following positive ions in the order in which they will be discharged first in preference to others.
Zn2+, H+, Mg2+, Ca2+, K+, Fe2+, Cu2+, Na+ .
(b) Arrange the following ions in the order in which they will be discharged first in preference to others.
Br–, Cl–, SO42– NO1-3 , I–, OH–.
Ans. (a) Cu2+, H+, Fe2+, Zn2+, Mg2+, Na+, Ca2+ , K+
(b) OH–, I–, Br–, Cl–, NO1-3, NO1-3 , SO2-4
13. Pick out the cations and anions from the following:
SO42- , Pb2+, NH4+ , OH–,
NO1-3 , H+, Ag+, Cu2+, CN–, Cl–.
Ans. Cations: Pb2+, NH4+, H+, Ag+ and Cu 2+
Anions: SO2-4, OH–, NO1-3, CN–, and CI–,
14. Choose the correct answer from the choices (i), (ii), (iii) and (iv).
(a) In the electrochemical series, the reducing character of the elements from top to bottom:
i) increases ii) decreases
iii) remain unchanged iv) none of these
(b) Which one of the following compound will decompose when electricity is passed through their aqueous solutions?
i) Sugar ii) Urea
iii) Copper sulphate iv) Ethyl alcohol
(c) The flow of current in an electrolyte is due to the movement of:
i) electrons ii) protons
iii) ions iv) molecules
(d) The compound which has only molecules in its solution
i) acidified water ii) Ammonium hydroxide
iii) Sugar solution iv) Salt solution
(e) The ratio of hydrogen and oxygen formed at the electrodes during electrolysis of acidified water.
i) 1:1 ii) 2 : 1
iii) 1 : 2 iv) 2 : 2
Ans. a) (ii) b) (iii) c) (iii) d) (iii) e) (ii)
15. Give reasons :
(a) We cannot store AgNO3 solution in Cu vessels.
Ans. Because Cu being more reactive than Ag will displace silver (Ag) from its solution.
(b) Can we store CuSO4 solution in a Zn vessel? Give a reason.
Ans. No, because Zn being more reactive than Cu, will displace Cu from its solution.
(c) Which of the two Zn and Cu would occur more readily in nature as a metal and which as an ion? Explain.
Ans. Zn will exist as anion and Cu as a metal. Because Zn is more reactive than Cu and is placed higher in the electrochemical series. So zinc has more tendency to lose electrons and form positive ion.
(d) Hydroxyl ions (OH)– are lower in the electrochemical series than chloride ions (Cl)– yet, when a concentrated solution of hydrochloric acid is subjected to electrolysis, the hydroxyl ions do not get discharged. Give reason.
Ans: According to the position in electrochemical series, hydroxyl ions should be discharged in preference to the chloride ions. However, in this case, it is the concentration of ion that determines the discharge of ions at
electrodes. Hence, chloride ions which have a greater concentration are discharged in preference to hydroxyl ions which have lower concentration.
(e) While electrolysing concentrated H2SO4, the bulb glows very dimly but when dilute, the bulb glows brightly. Give reason.
Ans. Concentrated (99%) sulphuric acid behaves like a weak electrolyte as it has very little hydronium ions in it. But when diluted by adding water, it produces a large number of hydronium ions. Now it behaves like a stronger
electrolyte and hence, the bulb glows brightly.
(f) Why aluminium is extracted from its oxide by electrolysis and not by reducing agents?
Ans. Aluminium oxide (Al2O3) is a highly stable oxide, as Al has a strong affinity for oxygen, hence conventional reducing agents like carbon, carbon monoxide and hydrogen cannot reduce Al2O3 to aluminium. Hence aluminium is
extracted by electrolysis.
(g) State giving reasons, in what state or medium does NaCl conduct electricity?
Ans. NaCl is an ionic compound, its ions Na+ and Cl– are strongly bonded by electrostatic forces of attraction. Free ions which are required for conducting electricity can be produced by heating NaCl strongly. Thus the molten state of NaCl will conduct electricity.
(h) State giving reasons, in what state or medium does HCl gas conduct electricity.
Ans. HCl gas when dissolved in water conducts electricity. HCl in the gaseous state is unionised and does not conduct electricity. When it is dissolved in water which is a polar solvent. HCl is ionised to H+ & Cl–.
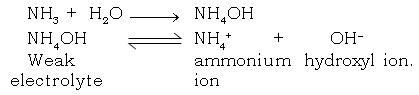
(i) State giving reasons, in what state or medium does NH3 gas conduct electricity.
Ans. NH3 gas when mixed in water conducts electricity. NH3 gas is unionised in the gaseous state, but aqueous solution of it, forms a weak electrolyte. Which dissociates into ammonium and hydroxyl ions
(j) Hydrogen is a non – metal even then it is included in the electrochemical series of metal. Explain.
Ans. Electrochemical series of metals involves those elements which can lose electrons. Hydrogen also shows electropositive behaviour (tendency to loose electrons) therefore it is placed in the series.
(k) Lead bromide undergoes electrolytic dissociation in the motlen state but is a non electrolyte in the solid state.
Ans. In solid lead bromide, lead and bromide ions are held together by strong electrostatic force of attraction and hence are not free for conduction of electricity. On melting, lead bromide dissociates into Pb2+ and Br1– ions
which conducts electricity.
(l) Carbon tetrachloride is a liquid but does not conduct electricity.
Ans. Carbon tetrachloride is a non-polar covalent compound. It does not dissociate to form ions and hence it does not conduct electricity.
(m) Give a reason why the metals – copper, silver and lead are electro refined but – K, Na and calcium are not.
Ans. Metals like K, Na and Ca are obtained/extracted by electrolysis which produce metals in the pure form without the need for further refining. Metals like copper, silver and lead are to be electro refined as they are extracted from other processes in which they are obtained with traces of impurities in it.
(n) During electrolysis of copper sulphate solution traces of dil. H2SO4 is added
Ans. Dilute H2SO4 enhances the conductiviy of the electrolyte & may prevent the hydrolysis of the electrolyte.
16, Answer the following :
(a) State on what basis are acids, bases and salts classified as strong and weak electrolytes.
Ans. On the basis of degree of dissociation of compounds (acids, bases and salts) in the aq. or molten state, they are classified as strong and weak electrolytes.
(b) State how activity series of metal plays a role in extraction of metals from their oxides.
Ans. Metals which are at the top of activity series (like K, Na, Ca, Mg and Al) are extracted by electrolysis of their fused salts. Their oxides are highly stable and hence do not decompose thermally and conventional reducing agents
like carbon monoxide and H2 cannot reduce them. Metals below Al in the activity series such as zinc, iron, lead etc can be extracted from their oxides by reduction using conventional reducing agents. Metals like Hg and Ag can be extracted from their oxides by thermal decomposition.
(c) Give one example in each case of a substance which contains:
a) ions only
b) molecule only
c) both ions and molecules
Ans. a) Hydrochloric acid(HCl)
b) Alcohol, pure water
c) Acetic acid (CH3COOH)
(d) Electrolysis is used in the purification of metals. Name the:
a) anode.
b) cathode and
c) electrolyte.
used to get pure copper from a piece of impure copper.
Ans. a) Piece of impure copper
b) Thin sheet of pure copper
c) Aqueous copper sulphate
(e) In this question you are required to use the word/words that will make each sentence into correct statement which is to be written down in full.
a) In an electrolyte, the number of positive ions is equal to the number of negative ions.
b) During electrolysis, oxidation occurs at the cathode and reduction occurs at the anode.
c) When dilute H2SO4 is electrolysed sulphur is precipitated at the anode.
Ans. a) In an electrolyte, the total positive charge is equal to the total negative charge.
b) During electrolysis, oxidation occurs at the anode and reduction occurs at the cathode.
c) When dilute H2SO4 is electrolysed oxygen is evolved at the anode.
(f) Consider that the following ions to be present in an electrolyte:
Na+, K+, H+, SO42–, Cl– and OH–.
If platinum electrodes are used in its electrolysis:
a) name the ions that would be discharged at the anode.
b) name the ions that would be discharged at the cathode.
c) give a reason to support your answer in (a) and (b).
d) Summarize the ionic reaction taking place at the cathode and at the anode.
Ans. a) Hydroxyl ions (OH–)
b) Hydrogen ions(H+)
c) The preferential discharge of ions at the electrodes is determined by their position in the electrochemical series. The ion which is placed below in the series is discharged in preference to those placed above it. OH– ion is placed below SO42– and Cl– ions. Hence, OH– ions gets discharged at the anode. In the same way, H+ ion is placed below K+ and Na+ ions. Hence, H+ ions gets discharged at the cathode.
d) Reaction at the cathode:
H+(aq) + e – → H × 4
H + H → H2 × 2
Reaction at the anode:
4OH– – 4e– → 2H2O + O2
g. a) Mercury is a liquid and allows the flow of electricity through it but is not an electrolyte. Give reason.
b) Name the process by which an electrovalent compound breaks up into free mobile ions in the molten or aqueous form
Ans. a) An electrolyte is a substance which on dissolving in water or melting breaks up into positively and negatively charged ions. But mercury is a metal, so on dissolving in water or in liquid state. It cannot break up into cations and anions. When an electric current passes through mercury, it does not undergo any decomposition and no new substance is formed. Electric current passes though mercury due to the presence of free electrons and not due to the formation of ions. Hence, mercury is a metallic conductor and not an electrolyte.
b) Dissociation
17. Complete the table given below :
(i) Electroplating an iron rod with silver.
(ii) Electroplating a copper sheet with nickel.
(iii) Electro refining of silver.
(iv) Extraction of potassium from KCl.
(v) Extraction of aluminium from Al2O3.
Ans.

18. Copy and complete the following table in reference to the electrolysis of water acidified with sulphuric acid:
Ans.

EQUATION SUMMARY
Electrolysis
a. Electrolysis of fused lead bromide.
Electrode reaction
Dissociation of PbBr2

b. Electrolysis of acidified water.
Electrode reaction
Dissociation of water (acidified)

c. Electrolysis of aqueous copper sulphate (active copper electrodes)
Electrode reaction :
Dissociation of aqueous of CuSO4
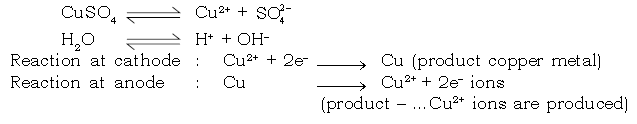
Electrolysis of CuSO4 (aqueous) with inert Platinum electrodes
Electrode reaction :
Dissociation of aq CuSO4
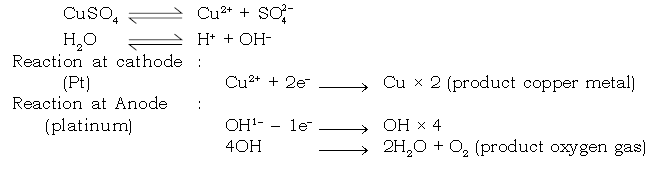
Application of Electrolysis Electroplating of an article with Nickel
Electrode reaction :
Dissociation of aqueous Nickel Sulphate

Electroplating of an article with silver
Electrode reaction :
Dissociation of Sodium Silver cyanide

Electro–refining of Copper
Electrode Reaction :
Dissociation of aqueous Copper sulphate
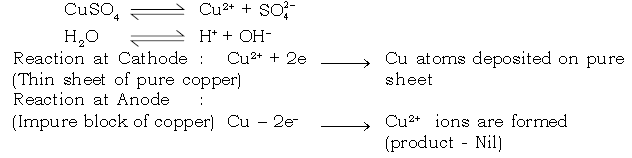
Electrometallurgy :
Extraction of sodium metal
Dissociation of sodium chloride fused

Extraction of potassium metal
Dissociation of fused potassium bromide :

Extraction of calcium metal.
Dissociation of fused calcium chloride
Extraction of Aluminium
Dissociation of pure alumina