Students of ICSE Class 10 should refer to Metallurgy ICSE Class 10 Chemistry Questions below which have come in past board exams. You should always go through questions that have come in previous years. This will help you to understand the pattern of questions in ICSE Class 10 Chemistry and prepare accordingly. This will help you to get better marks in ICSE Class 10 Board Exams
ICSE Class 10 Chemistry Important Questions Metallurgy
Metallurgy is an important chapter in ICSE Class 10 Chemistry. Our faculty has prepared the following ICSE Class 10 Chemistry Questions and answers based on the latest syllabus and books issued for the current academic year. Please refer to the solved questions below and also see links provided for other chapters.
Metallurgy ICSE Class 10 Chemistry Questions
Metallurgy ICSE Class 10 Chemistry Questions
DO WE KNOW ?
Metals lose electrons and form positive ions easily.
Metals have 1, 2 or 3 electrons in the valence shell and are placed on extreme left of the periodic table.
Non – metals gain electrons and form negative ions easily.
Non – metals have 4, 5, 6 or 7 electrons in the valence shell and are placed on the right of the periodic table.
Metals and non-metals can be differentiated on the basis of their basic atomic structure. Few metals like gold, copper, platinum occur in free state as they are unreactive in nature.
Most of metals are found in the combined state in the form of compounds.
The compounds are in the form of sulphates, sulphides, oxides, Halides, carbonate etc (ores). Thus the majority of the metals have to be extracted from their ores, through the process known as metallurgy.
A. INTRODUCTION
1. Define the following terms
i. Metal
ii. Non – metal
iii. Alkali metal
iv. Alkaline earth metal
Ans. i. Metal : It is an element which tend to form positive ions (cations) on ionization by losing one or more electrons for eg. Sodium, calcium etc.
ii. Non-metal : It is an element which tend to form negative ions (anions) on ionization by gaining one or more electrons for eg. Chlorine, Nitrogen etc.
iii. Alkali metals : Metals of IA group having one electron in valence shell which is easily lost (forming cations). They are placed on extreme left of the periodic table, are light and highly electropositive. E.g. Li, Na, K.
iv. Alkaline earth metals : Metals of II A group having 2 electrons in valence shell. They are placed on left side of the periodic table, are light and electropositive in nature. E.g. Be, Mg.
2. Name the metallic elements showing the following properties.
a. Metal which is liquid at room temperature.
Ans. Mercury, gallium.
b. Metal which is neither malleable nor ductile.
Ans. Mercury, potassium and sodium.
c. Metal which is soft and heavy.
Ans. Lead.
d. Metal which can be cut with a knife.
Ans. Sodium
e. Hard but lacks mechanical strength.
Ans. Magnesium
f. Is brittle at ordinary temperature.
Ans. Zinc.
g. Forms a liquid alloy at ordinary temperature.
Ans. Sodium.
h. Is soft and floats on water.
Ans. Sodium, potassium.
i. Is trivalent and forms an acidic oxide.
Ans. Chromium
j. Is trivalent and forms an amphoteric oxide.
Ans. Aluminium.
k. Metal with low boiling point.
Ans. Mercury.
l. Is difficult to extract from its ore.
Ans. Sodium and Potassium.
m. Has a low melting point.
Ans. Sodium and Potassium.
3. Name the non-metal which shows the following properties.
a. Is dense and sublimes on heating.
Ans. Iodine.
b. Which is lustrous.
Ans. Iodine.
c. Conducts electricity in the solid state.
Ans. Graphite.
d. Is liquid at ordinary temperature.
Ans. Bromine.
e. Forms stable compound with hydrogen.
Ans. Chlorine.
f. Forms an acidic and a neutral oxide
Ans. Nitrogen, Carbon.
General characteristics of metals and non metal.
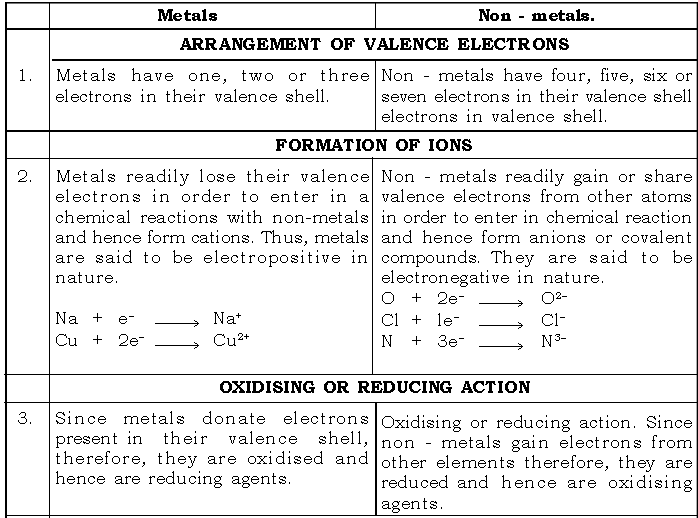
4. Distinguish between metals and non – metals.
a. Physical properties.


(b) Chemical Properties.


5. Bring out differences between metals and non – metals with respect to :
i. arrangement of valence electrons ii. formation of ions
iii. oxidising or reducing action iv. nature of oxides
Ans.

B. METAL ACTIVITY SERIES
6. What is activity series of metals?
Ans. The arrangement of metals in the decreasing order of their reactivity is called activity series of the metals.
7. Why is hydrogen placed in the acitivity series of metals?
Ans. Hydrogen has one electron in its valence shell and can lose it like metal atoms.
8. Comment about the reactivity of the metals depending on their position in the reactivity series.
Ans. Metals at the top of the reactivity series are more reactive and metals at the end of the series are least reactive.
9. Why does the reducing power of metals decrease on moving down the acitivity series?
Ans. As we go down the activity series, the ability of the element to lose electrons goes on decreasing, since reducing power depends on the ability of an element to lose electrons, the reducing power decreases as we go down in the metal activity series.
C. METALLURGY
10. Name two different metallic compounds in each case which occur as it halide (ii) oxide (iii) sulphides
Ans. (i) Cryolite (Na3 Al F6), Fluorspar (CaF2), Hornsilver (AgCl Rock salt (NaCl)
(ii) Bauxite (Al2O3 2H2O), Zincite [ZnO]
(iii) Iron Pyrite (FeS2), zinc blende (ZnS), Galena (Pbs), cinnabar (HgS).
11. Define the following terms used in metallurgy
a. Metallurgy b. Mineral c. Ore
d. Matrix e. Flux f. Smelting
Ans. a. Metallurgy : The large scale process involved in the extraction of pure metal from their respective ores is called metallurgy.
b. Mineral : The compounds of various metals found in nature associated with other earthly impurities are called minerals.
c. Ore : The naturally occuring minerals form which metals can be extracted profitably and conveniently are called ores.
d. Gangue or Matrix : The rocky impurities including silica (SiO2), mud etc associated with the ore is called matrix or gangue.
e. Flux : The substances added to the ore to get rid of the matrix (earthly impurities) resulting in the formation of a fusible compound slag.
f. Smelting : Smelting is the process of reducing the roasted oxide ore and removing the gangue with the help of an appropriate flux added with the ore. It reduces the oxide ore to metals in the molten condition.
12. (i) Differentiate between mineral & orc
(ii) Matrix & flux
Ans.

13. Give the common ores of Aluminium, Zinc and Iron.
Ans. Ores of Aluminium.

14. What are the different process involved during metallurgy.
Ans. The different process involved for obtaining pure metal from impure ore are :
(i) Concentration of ore for removal of impurities.
(ii) Conversion of concentrated ore to oxide.
(iii) Reduction process.
(iv) Refining of impure metal.
15. Name the different processes involved in the concentration of ore and briefly explain them.
Ans. ( i ) E l e c t r o m a g n e t i c m e t h o d / M a g n e t i c Separation: This process involves the separation of magnetic ore from non-magnetic gangue or magnetic gangue from non magnetic ore.

(ii) Froth floatation method : It involves the separation of ore and gangue by preferential wetting of ore by the oil (usually pine – oil), which forms the foam and hence floats on the top when water – oil mixture is agitated with compressed air.

(iii) Gravity separation or Hydraulic method : This hydraulic method is used for the ore and gangue which have different densities. The denser ore particles settle in grooves on a vibrating sloped table, while lighter gangue particles are washed down by water.

(iv) Chemical Method or Leaching : the one is treated with a suitable reagent (acid, base or some other reagent) such that the ore is soluble in it but the impurities are not. The impurities are removed by filtration. The solution of the ore, i.e. the filtrate is treated with a suitable reagent which precipitates the ore
16. Name the ores that can be concentrated by the following methods
(i) Magnetic separation (ii) Froth floatation
(iii) Gravity separation (iv) Leaching
Ans. (i) Ores of iron such as Haematite, Magnetite or iron pyrites.
(ii) Sulphide ores eg. zinc blende (ZnS) & Galena (PbS)
(iii) Oxide ores of Iron & tin
(iv) Ores of aluminium, silver & gold
17. Differentiate between Roasting and calcination.
Ans.

18. Write the main methods of obtaining a metal from its ore.
Ans. The three main methods of obtaining a metal from its ore are :
(i) Electrolytic reduction : It involves extraction by electrolysis of fused salts.
(ii) Chemical reduction : It involves extraction by reducing agents like (coke, carbon monoxide or H2).
(iii) Thermal decomposition : It involves heating the oxide ore, leaving a metallic residue.
19. a) What is refining of metals?
b) What factors does the method of purification depend on?
c) Describe in brief the different processes involved in refining of metals.
Ans. (a) It is the separation of the extracted metal from the residual impurity such as other metals, non-metals (eg. P, C, Si, etc.), unreacted oxides and sulphides of the metal.
(b) The method of purification used depends on :
(i) the nature of the metal.
(ii) nature of the impurites present in the metal.
(iii)Purpose for which the metal is to be used.
(c) (i) Distillation : Metals like mercury and zinc which are volatile distil over in pure form and the non-volatile impurity remains behind.
(ii) Liquation : Metals like lead and tin have low melting points, so they are heated on the sloping hearth of a furnace. The molten or fused metal flows away leaving behind the impurities.
(iii) Oxidation : Metals like iron which contain volatile impurities can be easily oxidised are purified by this method. The volatile oxides of phosphorus, sulphur and other impurities rise to the surface and are removed while the molten metal is left behind.
(iv) Electro-refining : The impure metal is made the anode, while a thin sheet of pure metal is made the cathode.
Electrolyte used is a salt solution of a metal, which is to be refined. Pure metal deposits at the cathode and impurities settle down forming anode mud.
20. (a) Name an ore of zinc
(b) How is the concentrated ore. changed to oxide?
(c) Explain using chemical equation the method used for reduction of the ore
(d) State the method used for refining zinc.
Ans. (a) Zinc is extracted from its ore, zinc blende (ZnS) or calamine (ZnCO3).
(b) The concentrated ore is converted to zinc oxide by roasting or calcination respectively.

(d) The impure zinc spelter on distillation or electrolytic refining gives pure zinc.
21. In the stages involved in the extraction of metals in general – give reasons for the following
(i) Dressing of the ore is an essential process in the extraction of metal from its ore.
(ii) In the froth flotation process, the ore floats on the top & the gangue settles down.
(iii) Concentrated ore converted to oxide.
(iv) Reduction of metallic oxides to metal in the extraction of metals from the ores- is based on the position of the metal in the activity series.
(v) State why aluminium is extracted from its oxide by electrolysis while copper, lead, iron by reducing agents and mercury and silver by thermal decomposition.
Ans. (i) Dressing is an important process, because it converts the impure ore to pure concentrated ore.
(ii) The ore is lighter and is wetted by oil, on agitation it forms foam with oil & floats on the top, whereas the gangue which is wetted by water, being heavy settles down.
(iii) To convert the pure concentrated ore to its oxide since oxides are easy to reduce.
(iv) Metals differ in tendency to lose valence electrons & hence can be arranged in a series according to their tendency to give up valence electrons.
(v) Aluminium is very electropositive, has strong affinity for oxygen. Thus its metallic oxide can be reduced by electrolysis. Metals copper, lead and iron are less electropositive and have less affinity for oxygen. Metallic oxides of these metals can thus be reduced by reducing agents i.e. C [coke], CO, H2. Metals Hg and Ag are least electropositive and have least affinity for oxygen. Metallic oxides can thus be reduced by heat alone.
D. Extraction of Aluminium
22. Answer the following questions with respect to Bayer’s process.
a. What is Baeyer’s process? or What is the process used for concentration of aluminium ore (Bauxite).
Ans. Baeyer’s process is the process for concentration of Bauxite ore. The impure baxuite is purified to pure aluminium through different steps.
b. What are the impurities present in bauxite?
Ans. Bauxite has iron (III) oxide (Fe2O3) and silica (SiO2) impurities.
c. What is red mud? How is it removed?
Ans. Red mud is the insoluble impurity such as iron (III) oxide etc. left behind on treating the ore with sodium hydroxide or caustic soda. The impurities are removed by dissolving the ore in sodium hydroxide followed by filtration.
d. Name the chemical used for concentration of bauxite. What is its function in Baeyer’s process.
Ans. Sodium hydroxide is used for concentration of bauxite. Bauxite is digested with conc. soln. of NaOH under pressure. Being amphoteric, it reacts with NaOH forming salt and H2O. Moreover the impurities present (Fe2O3 and SiO2) in bauxite remain unaffected with conc. alkali soln. Hence it is used to remove impurities in bauxite ore.
e. Give a brief account of the steps carried out in Baeyer’s process.
OR
Give the equations of the reaction in Baeyer’s process.
Ans. Baeyer’s process : It is the process for concentration of Bauxite ore.
The impure bauxite is purified to pure alumina through different steps.
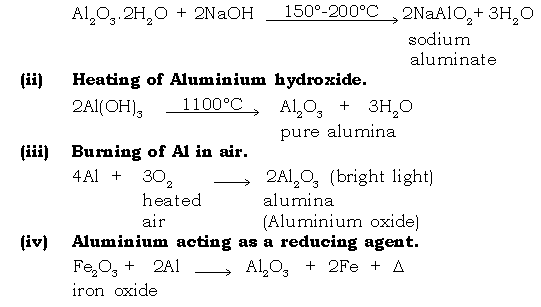
Step I : Conversion of impure bauxite to sodium aluminate.

Step II : Conversion of sodium aluminate to aluminium hydroxide.

Step III : Conversion of Al(OH)3 to pure alumina by heating it.

f. Why are the crystals of Al(OH)3 added to sodium aluminate and water in step II in Baeyer’s process?
Ans. Crystals of Al(OH)3 are added to activate precipitation of Al(OH)3 from dil. soln. of NaAlO2. The process is known as seeding.
23. How is impure bauxite concentrated by Hall’s process? Give chemical equations involved?
Ans. Concentration of Ore by Hall’s process : Removal of impurities involves following steps.
Step I : Conversion of impure bauxite into sodium aluminate by heating with sodium carbonate.

(impure bauxite)
Step II : Passage of CO2 into sodium aluminate solution resulting in precipitation of Al(OH)3
2NaAlO2 + 3H2O + CO2 → Na2CO3 + 2Al(OH)3 ↓
Step III : Ignition of dry Al(OH)3 at temp of 1100ºC to give pure alumina

24. Answer the following questions based on the process of obtaining pure aluminium from alumina.
a. Name the process of extraction of aluminium from alumina.
Ans. Hall Heroult’s process.
b. Why is electrolytic reduction done to obtain aluminium?
Ans. Aluminium is amongst the highly reactive metals in the acitivity series, it forms strong oxides which are difficult to be reduced to metal. Hence, electrolytic reduction is done to obtain aluminium from alumina.
c. What is the composition of the electrolyte.
Ans. The electrolyte contains 1 part by weight of fused alumina, 3 parts by weight of cryolite (Na3AlF6) and 1 part by weight fluorspar (CaF2).
d. What is the function of Cryolite and fluorspar in the above process?
Ans. Cryolite and fluorspar acts as a solvent for fused alumina (Al2O3). They also lower the fusion (melting) point of the mixture and increase the conductivity of the electrolytic mixture.
e. Give the equations of the electrolytic reactions taking place in the electrolyte.
Ans.

f. What is the nature of the electrodes?
Ans. Cathode : Inner gas carbon lining of the cell.
Anode : Thick carbon rods.
g. Give the reaction at electrodes.
Ans. At cathode :
2Al3+ + 6e– → 2Al
At anode :
3O2– – 6e– → 3 [O]
3 [O] + 3 [O] → 3O2
h. What are the products formed at cathode and anode?
Ans. At cathode : Pure Al metal
At anode : O2 gas
i. A layer of powdered coke is sprinkled over the electrolytic mixture.
Ans. It prevents the burning of carbon electrodes and it also prevents or minimizes heat loss by radiation thereby maintaining the temperature of electrolytic mixture.
j. The graphite anodes are periodically replaced during the electrolysis of fused alumina.
Ans. The O2 gas evolved at the anode oxidises carbon anode to carbon monoxide which burns giving CO2.
2C + O2 → 2CO
2CO + O2→ 2CO2
Carbon anode is hence consumed and replaced after a certain period of usage.
k. Electrolytic reduction of alumina is a continuous process. Give reason
Ans. • Pure aluminium is produced at the cathode & being heavier than than electrolyte settles down it is periodically removed through the outlet or tap hole at the base.
• As the concentration of Al2O3 falls – the resistance of the bath increases, indicated by a glow on the control lamp at this moment more alumina is added & the process continues.
l. Why electrolytic reduction of pure Al2O3 is difficult to conduct at the fusion temperature (melting point) of the electrolyte pure alumina?
Ans. Because fused Al2O3 is almost a non-electrolyte and has a melting point 2050ºC. To attain such high temperature and to maintain the molten state of aluminium a large amount of electrical energy is required. The liberated Al metal (melting point 660ºC) may also tend to volatilize out and get wasted, hence the fusion temperature has to be lowered.
25. Explain electrorefining of Aluminium by Hoope’s process.
Ans. Electrolytic reduction by Hall Heroult’s process produces about 99% pure Al which can be further refined by electrolysis through (Hoope’s process) to give 99.9% pure Al. Electrolytic cell for refining of Aluminium by Hoope’s
process consists of three layers.
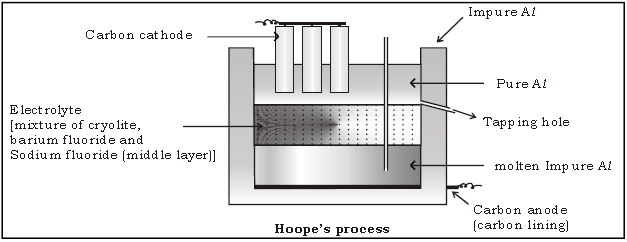
Anode : Molten impure Al at the bottom along with carbon lining.
Cathode : Molten Pure Al with carbon electrodes forming upper layer.
Electrolyte : mixture of cryolite, barium fluoride aluminium fluoride and calcium fluoride forming the middle layer.
Electrolysis : On passage of electric current Al3+ ions from middle layer are discharged at the cathode as pure Al and equivalent amount of Al from the bottom layer enters the middle layer.
Collection : Pure Al (about 99.9% pure) is withdrawn from the tapping hole.
26, For each substance listed below, explain its significance in the extraction of aluminium :
(a) Bauxite
Ans. Bauxite is the chief ore of Aluminium, it contains 60% Al2O3, the rest being S and & ferric oxide and hence can be used in the extraction of Aluminium.
(b) Sodium hydroxide
Ans. Bauxite dissolves in sodium hydroxide forming sodium aluminate, leaving behind insoluble impurities and thus concentrating the ore
(c) Cryolite
Ans. Cryolite lowers the fusion temperature from 2050C to 950°C & enhances the conductivity of electrolyte during electrolytic reduction
(d) Graphite
Ans. Graphite is used as anode during Electrolytic reduction of aluminium.
27. Give uses of Aluminium with reasons involved.
Ans. Uses of Al :
(i) Household use : Al metal is moulded into utensils.
Reason for use : It is light, cheap, corrosion resistant, good conductor of heat and is unaffected by food acids.
(ii) Paints : Al paints (Al powder + linseed oil) are generally applied on iron poles.
Reason : Aluminium layer prevents corrosion of Fe.
(iii) Packing : Al foil are used in food packing and photographic films.
Reason : It is resistant to corrosion, light metal has a bright appearance and is highly malleable
(iv) Electrical cable : Al is used in cable and transmission wires.
Reason : It is a good conductor of electricity.
(v) Mirrors : Al is used for making mirrors for reflecting telescope
Reason : If is an excellent refractive light.
(vi) Thermite welding : Al is used along with Fe2O3 to join broken process of Iron.
Reason : It is a powerful reducing agent.
28. What is thermite welding?(Gold schmidt’s aluminothermic process).
Ans. It is the welding process in which thermite mixture Fe2O3 + Al, (3 : 1) is used for welding broken ends of an iron girder. Metallic oxide (Fe2O3) is reduced with Aluminium. The process is also known as aluminothermy.
Fe2O3 + 2Al → Al2O3 + 2Fe + △
(reducing agent)
Reduced molten iron sinks and flows into the gap of the girder, thereby welding it.
29. Give reasons
a. Why the food containing iron salts’ should not be cooked in aluminium utensils?
Ans. Aluminium is more reactive than iron and can displace iron from its salt hence, food containing iron salts should not be cooked in aluminium utensils.
b. Aluminium is a more active metal than iron, but suffers less corrosion.
Ans. Aluminium on reacting with oxygen present in air forms aluminium oxide which forms a layer on the surface of aluminium and prevents it from further corrosion. On the other hand, rust formed on iron falls off easily exposing the iron for further corrosion.
c. Aluminium vessels should not be cleaned with powders containing alkalis.
Ans. Aluminium is an amphoteric metal it would react with the alkalis present in the cleaning powder to form a soluble salt and hence would corrode.
30. Define
a. Alloy b. Amalgam
Ans. a. Alloy is a substance prepared by adding other metals [or non-metals] to a base metal in appropriate corporations to obtain certain desirable properties. it is usually made by-melting the components together & solidifying the mixture.
b. Amalgam is an alloy in which the base metal is mercury.
e.g. Na/Hg [liquid amalgam], Zn/Hg amalgam . [used in voltaic cells]
31. Give the reasons for alloying.
Ans. Depending upon the purpose for which the particular alloy is used :
1. To modify appearance and colour.
e.g. aluminium bronze (Al + Cu) resembles gold as it is bright yellow.
2. To modify chemical activity
e.g. Sodium amalgam (Na + Hg) is less reactive than sodium.
3. To modify casting ability.
e.g. Type metal (Pb + Sn + Sb) expands on solidifaction and is easily cast.
4. To lower the melting point.
e.g. Solder (Pb + Sn) melts at 180°C which is lower than the melting point of lead or tin
5. To increase hardness, and tensile strength.
e.g. Brass (Cu + Zn) is harder than copper. Duralumin has a strength up to six times greater than pure aluminium.
6. To increase resistance to electricity
e.g. Nichrome (Ni + Fe + Cr) has more resistance (can produce much heat) to electricity copper.
32. Give the names, composition, properties and uses of alloys of following.
a. Copper and zinc
b. Aluminium and magnesium
c. Lead d. Iron
Ans.
a. Copper and zinc alloys
Brass : Cu , Zn
It is hard lustrous, easily cast, malleable, ductile.
Used in electrical fitting, decorative articles.
Bronze : Cu , Zn , Sn
It is hard, brittle, takes up polish.
Used in statues, medals, coins
German silver : Cu , Zn , Ni
It is hard, silvery, takes up polish
Used for making decorative articles.
Gun metal : Cu , Zn , Sn , Pb
It is hard, silvery, takes up polish.
Used in Barrels, cannon.
Bell metal : Cu , Sn
Brittle, more sonorous than Cu and Sn. Used in Bells, gongs etc.
b. Aluminium and magnesium alloys
Duralium : Al , Mg , Mn , Cu , Al imparts lightness and magnesium imparts strength. Light, strong, corrosion resistant.
Uses : Aircrafts, light tools, Pressure cooker.
Magnalium : Al , Mg Light, hard, tough, corrosion resistant.
Uses : Aircrafts, scientific tools
c. Lead alloys
Solder : Pb , Sn Sn lowers melting point of alloy
Uses : Electrical fuse and soldering purpose.
Type metal : Pb , Sn , Sb Sn and Sb lowers melting point enhances castability, enhances expansion capacity on solidification.
Uses : Printing blocks.
d. Iron alloys :
Stainless steel : Fe , Ni , Cr , C
It is lustrous, corrosion resistant strong, Ni, Cr imparts lustre, C imparts hardness.
Used in utensils, cutlery, auto mobile parts.
Nickel steel : Fe , Ni ,C It is elastic, Ni imparts high tensile strength.
Used in cables, aircraft parts.
Tungsten steel : Fe , W , C Very hard, Tungsten imparts hardness.
Used in High speed machine parts.
ADDITIONAL QUESTIONS
I. Name the following :
1. Allotropic modification of carbon and good conductor of electricity.
Ans. Graphite.
2. Suphide ore of mercury.
Ans. Cinnabar (HgS).
3. Common name for sulphide ore of zinc.
Ans. Zinc blende
4. The process of removal of gangue from ore.
Ans. Concentration.
5. A naturally occuring compound of metal from which metal is extracted cheaply, profitably and conveniently.
Ans. Ore.
6. The process by which sulphide ores are concentrated.
Ans. Froth floatation process.
7. The process of heating of ore in the presence of air.
Ans. Roasting.
8. The most common ore of aluminium.
Ans. Bauxite.
9. Metallic oxides which are reduced by aluminium.
Ans. Ferric oxide, chromium oxide.
10. An ore concentrated by magnetic separation.
Ans. Haematite, magnetite or iron pyrites or ores of iron.
11. The metals added to steel to make it stainless steel.
Ans. Chromium and nickel.
12. The metal extracted from calamine
Ans. Zinc.
13. Gas obtained when zinc blende is roasted.
Ans. Sulphur dioxide.
14. The process of coating thin layers of Zn over the surface of iron.
Ans. Galvanisation.
15. The metal which can be refined by distillation refining
Ans. Mercury or zinc.
16. Metal refined by liquation refining.
Ans. Lead or tin.
17. A metal which can be reduced easily by thermal decomposition of its oxide.
Ans. Mercury, Silver.
18. A metal which lacks ductility.
Ans. Zinc.
19. A non-metal used in making alloys.
Ans. Carbon.
20. A metal other than mercury present in liquid amalgam.
Ans. Sodium.
21. Name a non-metal that has a metallic lustre and sublimes on heating.
Ans. Iodine.
22. A black metallic oxide reduced to metal on heating with coke.
Ans. copper oxide
23. The compound which on ignition at elevated temperatures gives pure alumina.
Ans. Aluminium hydroxide
24. A metal other than magnanese, present in duralumin but not in magnalium.
Ans. Copper
25, A compound added to lower the fasion tempersutre of the electrolytic bath in the extraction of Al.
Ans. Cryolyte and Fluorspar
II. 1. Choose the correct answer from the choices (a), (b), (c) and (d).
a) Which one of the following alloys contains lead?
i) Duralumin ii) Solder
iii) Brass iv) Bronze
Ans.
Solder
b) Duralumin is used for:
i) Construction of aircrafts and ships
ii) resistance in heaters
iii) making coins
iv) soldering
Ans.
Construction of aircrafts and ships
c) Solder is an alloy of:
i) Ni, Fe and Cl ii) Sn and Pt
iii) Sn, Sb and Pb iv) Sn and Pb
Ans.
Sn and Pb
d) A substance used in metallurgy to remove rocky material is called:
i) slag ii) flux
iii) oxidizing silver iv) Magnalium
Ans.
flux
e) Which of the following does not contain aluminium?
i) Alnico ii) Duralumin
iii) German silver iv) Magnalium
Ans.
German silver
f) Which of the following metals does not give out hydrogen when reacted with dilute acids?
i) Iron ii) Zinc
iii) Copper iv) Manganese
Ans.
Copper
g) Which of the following is not correct for metals?
i) Metal have metallic lustre.
ii) Metals are non-ductile.
iii) Hardness of metals varies from metal to metal.
Ans.
Metals are non-ductile.
iv) All metal except mercury exist as solids at room temperature.
h) Magnalium is an alloy of:
i) Al and Mn ii) Al and Mg
iii) Fe and Ni iv) Al and Co
Ans.
Al and Mg
i) A metal whose oxide can be reduced by electrolysis.
i) Copper ii) Silver
iii) Sodium iv) Platinum
Ans.
Sodium
j) An amphoteric oxide
i) CaO ii) CO2
iii) Fe2O3 iv) Al2O3
Ans.
Al2O3
k) An alloy which does not contain copper
i) Brass
ii) stainless steel
ii) Duralumin
iv) Bell metal
Ans.
stainless steel
l) The common name of the ore of iron – whose chemical formula is Fe3O4.
i) Iron pyrites
ii) Magnetite
ii) Haematite
iv) Spathic iron ore
Ans.
Magnetite
m) The chemical name for chief ore of Aluminium.
i) Aluminium fluoride
ii) Aluminium oxide
iii) Sodium aluminium fluoride
iv) Hydrated aluminium oxide.
Ans.
Hydrated aluminium oxide.
n) The process of dressing of the ore which involves separation of ore
& gangue – due to preferential wetting.
i) Magnetic separation
ii) Hydrolytic method
ii) Froth floatation method
iv) Chemical method
Ans.
Froth floatation method
2. Complete the statements given below by filling the blank with correct word.
a. The metal which has a low melting point is ______________.
[Mg/K/Cu/Fe]
Ans.
K
b. The metal which can be cut with a knife is ______________.
[Cu/Al/Na/Zn]
Ans.
Na
c. The non-metal whose oxide is a neutral oxide ______________.
[sulphur/ nitrogen/phosphorus]
Ans.
nitrogen
d. The divalent metal whose oxide is reduced to metal by electrolysis of its fused salt is ______________.
[Al/Na/Mg/K]
Ans.
Mg
e . The metal whose oxide which is amphoteric is reduced to metal by carbon reduction ______________.
[Fe/Cu/Zn/Al]
Ans.
Zn
f. The impurity which separates out on addition of a conc. soln. of alkali to impure bauxite is ______________.
[PbO/ Fe2O3/CuO/ZnO]
Ans.
Fe2O2
g. During electrolytic reduction of pure alumina, Al3+ ions are discharged in preference to Na+ ions of cryolite and Ca+2 ions of fluorspar at the cathode because it is ______________ in the electrochemical series.
[higher / lower]
Ans.
lower
h. During electrolytic reduction of alumina, the inert electrode is ______________.to a neutral gas.
[reduced /oxidised]
Ans.
oxidised
i. Aluminium powder as a constituent of paints, prevents ______________.
[heat radiation/ formation of rust/conduction of electric current]
Ans.
formation of rust
j. Transmission wires are made of aluminium, since aluminium is ______________.
[resistant /a good conductor of heat/a good conductor of electricity]
Ans.
a good conductor of electricity
k. Aluminium is an important constituent metal in duralumin since it is ______________.
[a good conductor of heat:unaffected by food acids/light]
Ans.
light
l. In dry cells the zinc container acts as a/an ______________.
[anode/cathode]
Ans.
cathode
m. The metal other than aluminium present in both magnalium and duralumin is ______________.
[manganese /magnesium/copper]
Ans.
magnesium
n. Electrical fittings are generally made of ______________.
[German silver/brass/gun metal]
Ans.
brass
o. An alloy which is sonorous is ______________.
[duralumin/bell metal/ type metal]
Ans.
bell metal
p. Addition of ______________. to lead, lowers the melting point of the alloy solder.
[Sb /Sb./Sn]
Ans.
Sn
3. If ‘M’ is a metal.
a. It will form M2+ by electron _______ [gain/loss] and the metal M is ________ [oxidised/reduced].
Ans.
loss oxidised
b. Its compound MY2, is ________ [electrovalent/covalent].
Ans.
electrovalent
c. Its oxide is a/an _______. [acidic/basic/amphoteric] oxide.
Ans.
basic
4. If ‘N’ is a non-metal.
a. It will form N1– by electron ________ [gain/loss] and the non-metal N is [oxidised/reduced].
Ans.
reduced gain
b. Its oxide is a/an _________ [acidic/basic/amphoteric] oxide.
Ans.
acidic
c. Its ion, N1– will form a neutral atom at the ________. [cathode/anode].
Ans.
anode
d. Its valence shell will have __________. [1/7/2] electron/s.
Ans.
7
e . It is highly electronegative and a ________. [bad/good] conductor of heat.
Ans.
bad
III. Answer the following :
1. Following question are related with the metallurgy of Aluminium.
(i) Which solution is used to react with bauxite as a first step in obtaining pure aluminium oxide?
(ii) The aluminium oxide for the electrolytic extraction of Al is obtained by heating aluminium hydroxide, write the equation for this reaction.
(iii) Name the element which serves both as anode and cathode.
(iv) Write the equation for the reaction at cathode.
(v) Give the equation for the reaction at anode.
Ans. (i) Sodium hydroxide solution

(v) Reaction at Anode
(O2– – 2e–→ O) × 3
3(O) + 3(O) → 3O2
2. Al is extracted from its ore, Bauxite. The ore is first purified.
(i) Write 3 balanced equations for the purification of bauxite by Hall’s process.
(ii) Name a chemical used for dissolving aluminium oxide.
(iii) Write an equation for the reaction which takes place at the anode during the extraction of Al by electrolytic process.
(iv) Mention one reason for the use of Al in Thermite welding.
Ans. (i) (a) Al2O3.2H2O + Na2CO3 → 2NaAlO2 + 2H2O + CO2
(b) NaAlO2 + 2H2O → NaOH + Al(OH)3↓
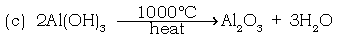
(ii) Fluorspar and cryolite act as a solvent. The percentage by weight
composition is as follows.
Alumina : 20%, Cryolite : 60% and Fluorspar : 20%
(iii) Reaction at anode.
3O2– – 6e– → 3(O)
3(O) + 3(O) → 3O2
(iv) As a reducing agent, aluminium has a high affinity for oxygen. It readily
removes oxygen from oxides of less reactive metals.
3. In order to obtain 1 tonne of aluminium, the following inputs are required.
4 tons of bauxite, 150 kg of sodium hydroxide and 600 kg of graphite.
The aluminium compound in bauxite is aluminium oxide and the main impurity is iron (III) oxide. Aluminium is obtained by the electrolysis of aluminium oxide dissolved in cryolite.
(a) When bauxite is treated with sodium hydroxide solution, what happens to:
(i) the aluminium oxide,
(ii) the iron (III) oxide.
(b) (i) Name the process used for the purification of bauxite.
(ii) Write the equation for the action of heat on aluminium hydroxide.
(c) (i) Write the formula of cryolite.
(ii) Write down the word which correctly completes the following sentence.
By dissolving aluminium oxide in cryolite a …….(conducting/non conducting) solution is produced.
(iii) Why is so much graphite required for the electrolytic process?
(iv) Write the equation for the reaction which takes place at cathode.
(d) In construction work, why is the alloy of aluminium duralumin used rather than pure aluminium?
Ans. (a) (i) Aluminium oxide forms sodium aluminate, a soluble solution
(ii) Iron (III) oxide remains unreacted and settles down.
(b) (i) Baeyer’s process

(c) (i) Na3AlF6
(ii) Conducting
(iii) Oxygen liberated at anode oxidises the graphite electrodes, thereby diminshing them. The graphite anode has to be replaced regularly and
hence lot amount of graphite is required for the electrolytic process.
(iv) 2Al3+ + 6e– → 2Al
d) Because, duralumin is light, strong and corrosion resistant.
4. Give reasons, why aluminium is used in :
(a) making alloys.
(b) wrapping chocolates.
(c) Painting electric and telegraphic poles.
(d) In aluminothermy.
(e) In making ships.
Ans. (a) It is strong, light and corrosion resistant.
(b) It is highly malleable
(c) It is corrosion resistant
(d) It is a good reducing agent
(e) Unaffected by sea water.
5. Name an alloy of :
(a) Aluminium used in aircraft construction.
(b) Lead used in electrical wiring or electrical work in joining metals.
(c) Copper in electrical appliances or household vessels.
(d) Iron used to make surgical instruments
Ans. (a) Duralumin
(b) Solder
(c) Brass
(d) Stainless steel.
6. Aluminium is used in thermite welding :
(a) What is thermite ?
(b) What is ignition mixture ?
(c) Write reaction for process.
Ans. (a) Thermit is a mixture of 3 parts of ferric oxide and one part of aluminium powder.
(b) Ignition mixture is a mixture of potassium chlorate and magnesium powder.
(c) Fe2O3 + 2Al Al2O3 + 2Fe + Heat
7. An ore on being heated in air forms sulphurous acid anhydride. Write
the process used for concentration of this ore.
Ans. Froth floatation
8. A metal M reacts with oxygen to form metallic oxide MO. This oxide reacts with moisture and carbon dioxide of the atmosphere to form a basic carbonate. Metal M prevents rusting of iron. Identify the metal M.
Ans: The metal M is Zinc
9. Give reasons :
a. Why do gold ornaments look new even after several years of use ?
Ans. Gold has low electropositivity as compared to other metals (It is placed below in the electrochemical series) hence it does not oxidise easily in air. Therefore gold ornaments looks new even after several years of use.
b. Copper oxide can be reduced using carbon but aluminium oxide cannot.
Ans. Aluminium is above copper in the reactivity series, it forms stronger oxides as compared to copper, hence copper oxide can be reduced using carbon but aluminium oxide cannot be reduced.
c. Sulphide or carbonate ores have to be converted to oxide before carrying out their reduction.
Ans. It is easier to carry out reduction of oxides, hence sulphide or carbonate ores have to be converted to oxide before carrying out their reduction.
d. A neutral gas other than oxygen which is formed at the anode during electrolysis of fused alumina.
Ans. Oxygen gas evolved at the anode oxidises graphite electrodes to from carbon monoxide a neutral gas formed at the anode.
e. Mercury is commonly stored in iron bottles.
Ans. Mercury does not from an amalgam with iron and hence is commonly stored in iron bottles.
10. Write balanced chemical equations for the following
(i) Conversion of impure bauxite to sodium aluminate in Baeyer’s process.

(vii) Reduction of zinc oxide.
ZnO + C → 14→ ºC → Zn(spelter) + CO2
(viii) Zn is placed in copper sulphate soln.
CuSO4 + Zn → ZnSO4 + Cu
(ix) Iron when treated with hot dil nitric acid.
3Fe + 8HNO3 → 3Fe(NO3)2 + 4H2O + 2NO(g)
(x) Iron is placed in silver nitrate soln.
2AgNO3 + Fe → Fe(NO3)2 + 2Ag
11. Match the properties and uses of metals or alloys in List 1 with the correct answer from List 2.

Ans. 1) C 2) E 3) D 4) A 5) B
13. What do you observe when,
a. Hydrogen is passed over heated copper oxide.
Ans. Black coloured copper oxide turns into a pinkish metal.
b. Iron/Zinc plate is kept in copper sulphate solution.
Ans. The blue colour of the copper sulphate disappears and a pinkish coloured metal is deposited on the plate.
IV. Differentiate between:
Electrometallurgy and Electrorefining.
Ans.

EQUATION SUMMARY
Extraction of Zinc :
Roasting :

zinc blende
Calcination

calamine /
zinc carbonate
Reduction of Zinc oxide

Extraction of Aluminium :
Baeyer’s process :

Chemical properties of Aluminium