ICSE Class 10 students can refer to ICSE Class 10 Metallurgy Notes given below which have been prepared as per the latest syllabus and guidelines issued by ICSE Board. All chapter wise revision notes for ICSE Class 10 Chemistry have been prepared by teachers have strong understanding of Chemistry. Read the notes prior to the exams to get better marks in exams
Metallurgy ICSE Class 10 Chemistry Revision Notes
Students can refer to the quick revision notes prepared for Chapter Metallurgy in Class 10 ICSE. These notes will be really helpful for the students giving the Chemistry exam in ICSE Class 10. Our teachers have prepared these concept notes based on the latest ICSE syllabus and ICSE books issued for the current academic year.
Revision Notes ICSE Class 10 Chemistry Metallurgy
Please refer to the detailed notes below
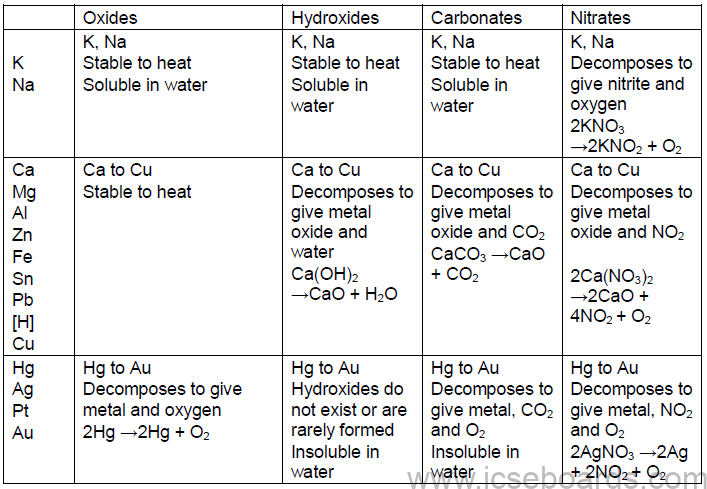
Metals and Non-metals
Position and characteristics of metals in the periodic table
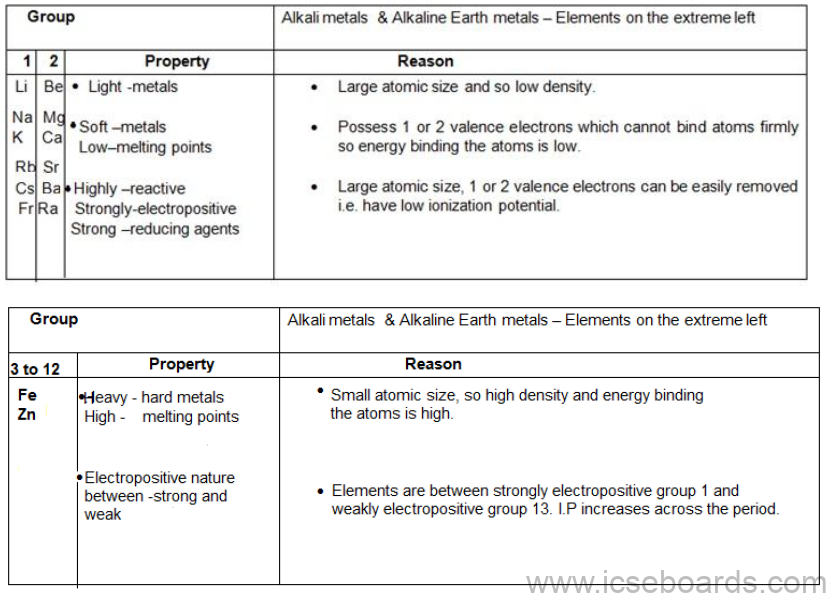

Characteristics of Alkali Metals and Alkaline Earth Metals

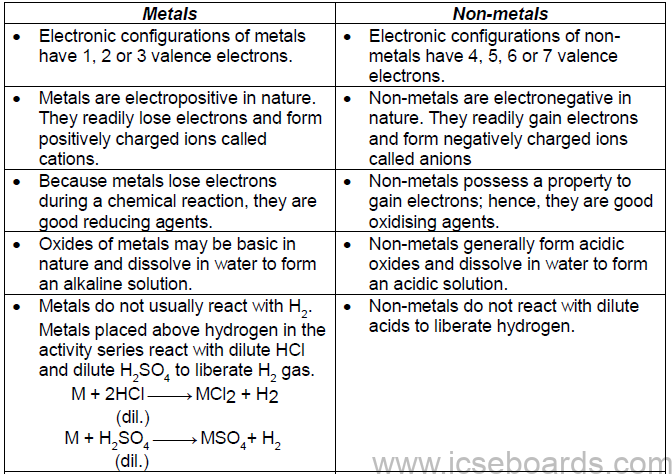
Comparison of Metals and Non-metals
• Physical Properties of Metals and Non-metals

• Chemical properties of metals and non-metals

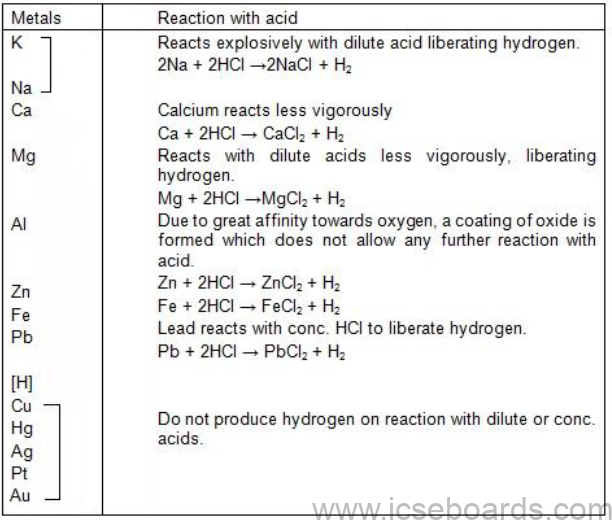
Study of Activity Series of Metals
Activity Series of Metals
Metals differ in tendency to lose electrons and hence can be arranged in a series according to their
tendency to give up valence electrons.
Activity Series
It is a series of metals arranged according to their decreasing reactivity.
The metals at the top of the series are
• Most easily oxidised
• Most electropositive
• Capable of displacing the metal below from its salt solution
Reactions of Metals
Characteristics of Metallic Compounds
Corrosion
When the surface of a metal is exposed to air, moisture or any other substance around it, the metal is said
to corrode, and the phenomenon is known as corrosion.
• Corrosion of Iron
Corrosion of iron is known as rusting.
Rusting is the slow oxidation of iron by atmospheric oxygen in the presence of water.
4Fe + 3O2 + 2xH2O →2Fe2O3.xH2O
• Conditions Necessary for Rusting
Presence of water (or moisture)
Presence of air (or oxygen)
• Prevention of Corrosion
Barrier protection: The process in which the metal surface is not allowed to come in contact with
atmospheric agents such as air or water is known as barrier protection.
For example, the metal (iron) is coated with another metal by using electricity in the process called
electroplating.
Sacrificial protection: The metal to be protected is covered with a more electropositive metal such
as zinc or magnesium. For example, iron is coated with zinc in the process called galvanisation.
Extraction of Metals Based on the Activity Series
Common terms used in extraction
• Metallurgy: The process used for the extraction of metals in their pure form from their ores is called
metallurgy.
• Minerals: The naturally occurring compounds of metals which are generally mixed with other matter
such as soil, sand, limestone and rocks are known as minerals.
• Gangue: Earthy impurities, including silica and mud, associated with the ore is called gangue.
• Ores: Those minerals from which metals are extracted commercially at a comparatively low cost and
with minimum effort are called ores.
• Flux: A flux is a substance which is added to the charge in a furnace to remove the gangue.
• Slag: It is the fusible product formed when flux reacts with impurities during the extraction of metals.
• Smelting: It is the process of reducing the roasted oxide ore and removing the gangue with the help of
an appropriate flux added with the ore.
Steps Involved in Extraction

Crushing and Grinding
Ores are crushed into a fine powder in big jaw crushers and ball mills. This process is called pulverisation.
Concentration of Ores
Gravity separation
Principle: Separation of
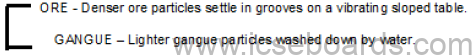
Process: The ore is poured over a vibrating sloped table with grooves, and a jet of water is allowed to
flow over it. The dense ore particles settle down in the grooves.
Magnetic separation
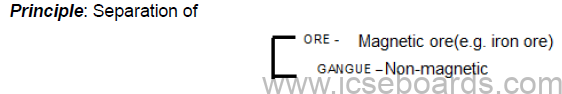
Process: The pulverised ore is placed on a conveyor belt. The magnetic particles are attracted to the
magnetic wheel and fall away separately from the non-magnetic particles.

Froth flotation
Principle: Separation of

Process: This method is generally applied for sulphide ores. The ore is taken in a large tank containing oil
and water and is agitated with a current of compressed air. The ore is wetted by the oil and separates
from the gangue in the form of froth.

Roasting and Calcination (If the ore is not an oxide)
A] Roasting
The process of heating the concentrated ore to a high temperature in the presence of excess air.
2ZnS + 3O2 → 2ZnO + 2SO2
B] Calcination
The process of heating the concentrated ore in the absence of air at a temperature not sufficient to
melt the ore.
ZnCO3 → ZnO + CO2
Reduction in Metallic Oxides
Reduction by electrolysis
Reduction in highly electropositive metals such as K, Na, Ca, Mg and Al oxides/halides.
Electrolysis of fused metallic salts
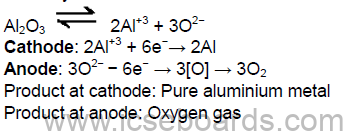
Reduction by reducing agents
ZnO + C → Zn + CO
2PbO + C → 2Pb + CO2
Refining of Impure Metal
1. Distillation: For refining volatile metals. Examples: Zinc, mercury
2. Liquation: For refining low-melting-point metals. Examples: Lead, tin
3. Oxidation: For refining metals by oxidation of their impurities. Example: Iron
4. Electrolytic refining: For refining impure metals by electrolysis. Examples: Cu, Al, Pb
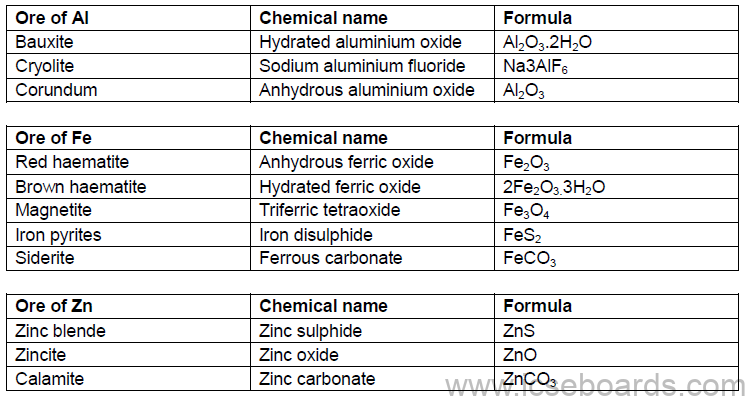
Common Ores of Aluminium Iron and Zinc
Extraction of Aluminium
Process for Extraction of Aluminium from Bauxite
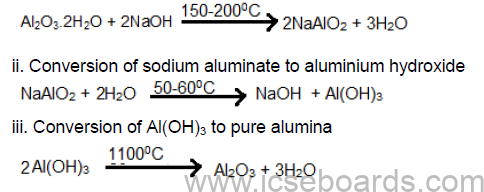
1. Concentration of Ore – Bayer process
i. Conversion of impure bauxite to sodium aluminate
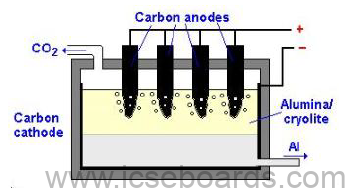
2. Electrolytic Reduction in Metallic oxide – Hall Heroult process
Electrolyte: Mixture of molten alumina 20%, cryolite 60% and fluorspar 20%.
Electrolytic cell: Rectangular steel tank with carbon lining.
Electrodes:
Cathode: Carbon lining (gas carbon)
Anode: Thick carbon (graphite)
Temperature: 950°C
Current: 100 amperes at 6–7 volts
Electrolytic reaction:
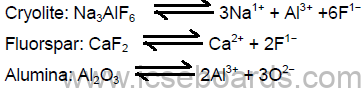
Cathode: 2Al3+ + 6e− → 2Al
Anode: 3O2− − 6e− → 3[O] →3O2
Products formed:
At cathode: Pure aluminium metal
At anode: Oxygen gas
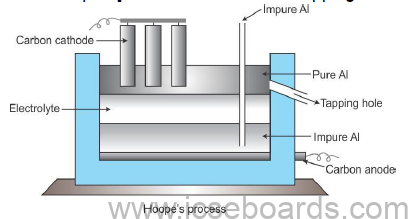
Refining of Aluminium (Hoope’s electrolytic process)
Tank contains three immiscible layers
Upper layer: Pure molten Al with carbon electrodes serves as cathode.
Middle layer: Mixture of cryolite, BaF2, AlF and CaF2 serves as the electrolyte.
Lower layer: Impure Al at the bottom along with carbon lining acts as the anode.
Electrolytic reaction:
Cathode: Al3+ + 3e− → Al
Anode: Al – 3e− →Al3+
Collection: Pure Al [about 99.9% pure] is withdrawn from the tapping hole.
Properties of Aluminium
Physical properties
Nature: Silvery light metal, malleable and ductile
Conductivity: Good conductor of heat and electricity
Boiling point: 2050°C
Melting point: 660°C
Chemical Properties
• Action with Air
4Al + 3O2 → 2Al2O3
2Al + N2 → 2AlN
• Action with Water
2 Al + 3H2O (steam) → Al2O3 + 3H2
• Action with Non-metals
2Al + 3Cl2 → 2AlCl3
2Al + 3S → Al2S
• Action with Alkalis
2Al + 2NaOH + 2H2O →2NaAlO2 + 3H2
• Action with Acids
2Al + 6HCl → 2AlCl3 + 3H2
2Al + 3H2SO4 (dil.) →Al2 (SO4)3 + 3H2
2Al + 3H2SO4 (conc.) →Al2 (SO4)3 + 6H2O + 3SO2
Nitric acid is rendered passive due to the formation of a thin aluminium oxide layer.
• Reducing Action
Reduces heated metallic oxides of Fe, Cr and Mn to metals.
Fe2O3 + 2Al → Al2O3 + 2Fe
Uses of Metals and Alloys
Uses of Aluminium
i. Being a strong, light and corrosion-resistant metal, it is used in alloys.
ii. Being a good conductor of electricity, it is used in the manufacture of cables for power transmission.
iii. Ships are made of alloys of aluminium because it is unaffected by sea water.
Uses of iron
i. Cast iron is used in drain pipes, gutter covers, weights and railings.
ii. Wrought iron is used in chains, horse shoes and electromagnets.
iii. Steel is used in the construction of buildings, overhead structures, machines and in various alloys.
Uses of Zinc
i. Mostly used for coating iron and steel sheets to prevent them from rusting.
ii. For making useful alloys such as brass, bronze and German silver.
iii. Zinc dust is used as a reducing agent for many organic reactions.
iv. Zinc compounds are used in paints, electroplast, preservatives for leather and a mordant for the
dyeing of textiles.
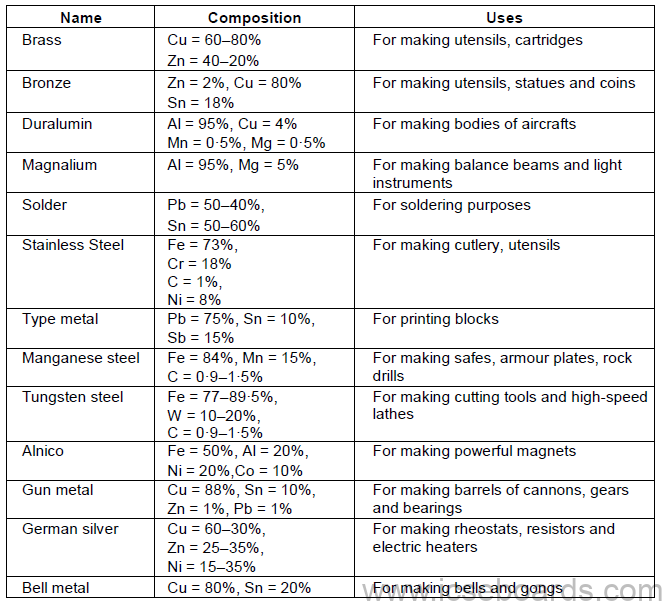
Alloys
An alloy is a homogeneous mixture of two or more metals or of one or more metals with
certain non-metallic elements.
• Reasons for Alloying
i. To modify appearance and colour
ii. To modify chemical reactivity
iii. To modify casting ability
iv. To lower the melting point
v. To increase hardness and tensile strength
vi. To increase resistance to electricity
Some Important Alloys and their Uses