Q1. (a) Define a chemical reaction.
(b) What happens during a chemical reaction?
(c) What do you understand by a chemical bond?
Answer:
(a) A chemical reaction is any alteration of matter that entails the creation of one or more compounds having wholly distinct characteristics.
b) Chemical bonds between atoms or molecules are broken during chemical reaction clusters of atoms in substances that are interacting together and atoms moving around to form new links to create new materials

(c) The attractive force that holds the atoms of a molecule together is known as a chemical bond in a compound.
Q2. Give one example each of which illustrates the following characteristics of a chemical reaction:
(a) evolution of a gas
(b) change of color
(c) change in state
Answer:
(a) When zinc and dilute sulfuric acid react. Evaporation of hydrogen gas occurs with effervescence.
Zn + H2SO4(dil) → ZnSO4 + H2
(zinc) (sulphuric acid) (zinc sulphate) (hydrogen)
(b) Black-colored copper sulphide is created when blue copper sulphate combines with hydrogen sulphide gas.
CuSO4 + H2S → CuS + H2SO4
(copper sulphate solution, blue) (hydrogen sulphide) (copper sulphide black solid) (sulphuric acid)
(c) Sulfur (solid) and hydrogen chloride are produced when hydrogen sulphide and chlorine (both gases) combine (gas).
H2S(g) + Cl2(g) → 2HCl(gas) + S(s)
(hydrogen sulphide) (chlorine) (hydrogen chloride) (sulphur yellow solid)
Q3. How do the following help in bringing about a chemical change?
(a) Pressure
(b) Light
(c) Catalyst
(d) Heat
Answer:
(a) When reactants are put under intense pressure, certain chemical reactions happen. For instance, when nitrogen and hydrogen are under high pressure results ammonia gas.

(b) Some chemical reactions can occur when light is present. Consider photosynthesis. For instance Photosynthesis

(c) A catalyst can speed up or slow down chemical processes, and some chemical reactions require one to adjust the rate of the reaction if it is too fast or sluggish.
1) Positive catalyst: In the production of ammonia from hydrogen and nitrogen, finely divided iron is utilized as a positive catalyst when a catalyst increases the pace of reaction.

2.) A catalyst that slows down a reaction is referred to as a negative catalyst. e.g. When lead nitrate is heated, it breaks into lead monoxide, nitrogen dioxide and oxygen.

(d) Some chemical reactions take place only in the presence of heat. e.g. When lead nitrate is heated, it breaks into lead monoxide, nitrogen dioxide and oxygen.
Q4. (a) Define catalyst.
(b) What are (i) positive catalysts and (ii) negative catalysts? Support your answer with one example for each of them.
(c) Name three biochemical catalysts found in the human body.
Answer:
(a) Catalyst: A catalyst is a substance that either speeds up or slows down a chemical reaction without changing chemically in the process.
(b) (i) Positive catalyst: A catalyst is referred to as a positive catalyst when it speeds up a chemical reaction. For instance, when potassium chlorate is heated to 700°C, it breaks down to produce oxygen gas; when MnO2 is added, the breakdown occurs at 300°C.

(ii) Negative catalyst: A catalyst is referred to be a negative catalyst when it slows down a chemical reaction. Example: The rate at which hydrogen peroxide breaks down is slowed down by the negative catalytic action of phosphoric acid. Alcohol also serves as a harmful catalyst in some chemical processes.
(c) Human body biochemical catalysts:
1.) Pepsin
2.) Tryspin
Q5. What do you observe when
(a) Dilute sulphuric acid is added to granulated zinc?
(b) A few pieces of iron are dropped in a blue solution of copper sulphate?
(c) Silver nitrate is added to a solution of sodium chloride?
(d) Ferrous sulphate solution is added to an aqueous solution of sodium hydroxide.
(e) Solid lead nitrate is heated?
(f) When dilute sulphuric acid is added to barium chloride solution?
Answer:
(a) Hydrogen gas with effervescence is produced when zinc combines with diluted sulfuric acid.
Zn + H2SO4 → ZnSO4 + H2
(b) When a small amount of iron is added to a blue copper sulphate solution, the solution’s blue hue gradually fades and eventually turns green.
(c) White insoluble ppt. of silver chloride is produced when a silver nitrate solution is mixed with a sodium chloride solution.
AgCl (ppt) + NaNO3 → AgNO3 (aq) + NaCl (aq) (aq)
(d) A murky green ferrous hydroxide ppt is produced when ferrous sulphate solution is mixed to sodium hydroxide solution.
Fe(OH)2 + Na2SO4 → Fe(OH)4 (aq) + 2NaOH (aq) (aq)
(e) When solid lead nitrate is heated, it breaks down into reddish-brown nitrogen and pale yellow solid lead monoxide.

(f) When few drops of dilute sulphuric acid is added to barium chloride solution, a white precipitate of barium sulphate is formed.

Q6. Complete and balance the following chemical equations:
(a) N2 + O3 →
(b) H2S + Cl2 →
(c) Na + H2O →
(d) NaCl + AgNO3 →
(e) Zn + H2SO4 (dil) →
(f) Fe SO4 (aq.) + NaOH (aq.) →
(g) Pb(NO3)2 →
(h) BaCl2(aq.) + H2SO4(aq.) →
Answer:
(a) N2 + O3 → 2NO(g)
(b) H2S + Cl2 → 2HCl + S
(c) Na + H2O → 2NaOH + H2↑
(d) NaCl + AgNO3 → AgCl + NaNO3
(e) Zn + H2SO4 (dil) → ZnSO4 + H2
(f) Fe SO4 (aq.) + NaOH (aq.) → Fe(OH)2+Na2SO4
(g) Pb(NO3)2 → PbO + 4NO2 + O2↑
(h) BaCl2(aq.) + H2SO4(aq.) → BaSO4 ↓ + 2HCl
Exercise – II
Q1. Fill in the blanks:-
(a) A reaction in which two or more substances combine to form a single substance is called a _______________ reaction
(b) A _______________ is a substance which changes the rate of a chemical reaction without undergoing a chemical change.
(c) The formation of gas bubbles in a liquid during a reaction is called _______________.
(d) The reaction between an acid and a base is called _______________.
(e) Soluble bases are called _______________.
(f) The chemical change involving iron and hydrochloric acid illustrates a _______________ reaction.
(g) In the type of reaction called _______________ two compounds exchange their positive and negative radicals’ _______________ respectively.
(h) A catalyst either _______________ or _______________ the rate of a chemical change but itself remains _______________ at the end of the reaction.
(i) The chemical reaction between hydrogen and chlorine is a _______________reaction
(j) When a piece of copper is added to silver nitrate solution, it turns _______________ in color.
Answer:
(a) A reaction in which two or more substances combine to form a single substance is called a combination reaction
(b) A catalyst is a substance which changes the rate of a chemical reaction without undergoing a chemical change.
(c) The formation of gas bubbles in a liquid during a reaction is called effervescence.
(d) The reaction between an acid and a base is called neutralization reaction.
(e) Soluble bases are called alkalis.
(f) The chemical change involving iron and hydrochloric acid illustrates a displacement reaction.
(g) In the type of reaction called double decomposition reaction, ions two compounds exchange their positive and negative radicals’ ions respectively.
(h) A catalyst either increases or decreases the rate of a chemical change but itself remains unchanged at the end of the reaction.
(i) The chemical reaction between hydrogen and chlorine is a combinaton reaction
(j) When a piece of copper is added to silver nitrate solution, it turns blue in color.
Q2. Classify the following reactions as combination, decomposition, displacement, precipitation and neutralization. Also balance the equations.

(b) Zn(s) + H2SO4 → ZnSO4(s) + H2(g)
(c) AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3
(d) NH3(g) + HCl(g) → NH4Cl(s)
(e) CuSo4(aq) + H2S(g) → CuS(s) + H2SO4(l)
(f) Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
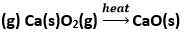
(h) NaOH + HCl → NaCl + H2O
(i) KOH + H2SO4 → K2SO4 + H2O
Answer:
(i) Combination Reactions:
(d) NH3(g) + HCl(g) → NH4Cl(s)

(ii) Decomposition Reaction:

(iii) Displacement Reaction:
(f) Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
(b) Zn(s) + H2SO4 → ZnSO4(s) + H2(g)
(iv) Precipitation Reaction:
(c) AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3
(e) CuSO4(aq) + H2S(g) → CuS(s) + H2SO4 (l)
(v) Neutralization Reaction:
(h) NaOH + HCl → NaCl +H2O
(i) 2KOH + H2SO4 → K2SO4 +H2O
Q3. Define:
(a) Precipitation
(b) Neutralization
(c) Catalyst
Answer:
(a) Precipitation: A chemical reaction in which two compounds in their aqueous state react to form an insoluble salt as one of the product.
Example:
BaCl2(aq) + Na2SO4(aq) → BaSO4– + 2NaCl(aq)
(Barium chloride) (Sodium sulphate) (Barium sulphate white ppt) (sodium chloride)
(b) Neutralization: A chemical reaction in which a base or an alkali reacts, with an acid to produce a salt and water only.
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O
(Sodium hydroxide) (hydro chloride acid) (Sodium chloride) (Water)
(c) Catalyst: A catalyst is a substance that either increases or decreases the rate of a chemical reaction without itself undergoing any chemical change. Here iron act as a catalyst and increases the rate of chemical reaction.

Q4. Explain the following types of chemical reactions giving two examples for each of them.
(a) Combination reaction
(b) Decomposition reaction
(c) Displacement reaction
(d) Double decomposition reaction
Answer:
(a) Combination reaction: A combination reaction is a chemical reaction in which two or more chemicals combine to generate a single substance.
A + B → AB
For instance,
(i) When iron and sulphur are heated together, iron sulphide is created.

(ii) When carbon combines with oxygen to create carbon dioxide, a gaseous substance.

b) Decomposition reaction: A process where a substance disintegrates as a result of the Decomposition reaction is the application of heat to two or more simple compounds. For instance, it heated mercury oxide breaks down into the components mercury and oxygen.

(ii) When heated, CaCO3 breaks down into carbon dioxide and calcium oxide.

(c) Displacement reaction: A reaction in which a more active element displaces a less active element from a compound is called displacement reaction.
AB + C → CB + A
For instance,
(i) Zinc, displaces copper from copper sulphate solution.
Zn + CuSO4(aq) → ZnSO4(aq) + Cu
(ii) Iron piece when added to copper sulphate solution, copper is displaced.
Fe + CuSO4 → FeSO4 + Cu
(d) Double decomposition reaction: A double decomposition reaction is a chemical process in which two compounds swap ions while still in their aqueous states to create new molecules.
AB + CD → CB + AD
For instance,
(i) AgNO3 + HCl → AgCl + HNO3(aq)
(ii) NaOH (aq) + HCl (aq) →NaCl (aq) + H2O
Q5. Write the missing reactants and products and balance the equations.

Answer:

Q6. How will you obtain?
(a) Magnesium oxide from magnesium
(b) Silver chloride from silver nitrate.
(c) Nitrogen dioxide from lead nitrate.
(d) Zinc chloride from zinc.
(e) Ammonia from nitrogen.
Also give balanced equations for the reactions.
Answer:
(a) Magnesium when burnt in air in presence of oxygen magnesium oxide is formed.

Q7. What do you observe when:-
(a) Iron nail is kept in copper sulphate solution for sometime.
(b) Phenolphthalein is added to sodium hydroxide solution.
(c) Blue litmus paper is dipped in dilute hydrochloric acid.
(d) Lead nitrate is heated.
(e) Magnesium ribbon is burnt in oxygen.
(f) Ammonia is brought in contact of hydrogen chloride.gas.
Answer:
(a) An iron nail develops a brown covering of copper. Chemical reaction is the cause of this.
CuSO4 (aq) + Fe (s) FeSO4 (aq) + Cu (s)
(b) Solution turns pink.
(c) Blue litmus reacts with acid and turns red.
(d) The colorless gas is oxygen, the reddish-brown gas is nitrogen dioxide, and the pale yellow solid is lead monoxide.

Q8. Give reason:
(a) A person suffering from acidity is advised to take an antacid.
(b) Acidic soil is treated with quick lime.
(c) Wasp sting is treated with vinegar.
Answer:
(a) An antacid reduces stomach acidity.
(b) To neutralize acidic soil, it can be treated with a base, such as quick lime.
(c) Because vinegar is a weak acid, it can be used to neutralize the alkaline nature of wasp stings.
Q9. What is meant by the metal reactivity series? State its importance, (any two points).
Answer:
The metal reactivity series is a list of metals categorized according to decreasing order of chemical reactivity. Unique characteristics of the activity series:
1. From potassium to gold, the ease with which a metal in solution loses electron(s) and produces a positive ion decreases.
2. The activity series includes hydrogen because, like metals, it too loses an electron and turns positively charged (H+) throughout the majority of chemical reactions.
3. The series makes it easier to compare metals in terms of how reactive they are.
4. It is also simple to compare the metals’ compounds, including their oxides, carbonates, nitrates, and hydroxides.

Q10. What are oxides?
Give two examples of each of the following oxides.
(a) Basic oxide
(b) Acidic oxide
(c) Amphoteric oxide
(d) Neutral oxide
Answer:
An oxide is a compound which essentially contains oxygen in its molecule, chemically combined with a metal or a non-metal.

Q11. Define exothermic and endothermic reactions. Give two examples of each.
Answer:
Exothermic Reactions:- Exothermic reactions are those in which heat is released chemically. The temperature rises as a result.
Example:
(i) When carbon burn in oxygen to form carbon dioxide, a lot of heat is produced.
C + O2 → CO2 + heat
(ii) When water is added to quicklime a lot of heat is produced which boils that water.
CaO + H2O → Ca (OH)2 + heat
Endothermic Reactions:- Endothermic reaction refers to a chemical process in which heat is absorbed. It lowers the temperature.
Example:
(i) When nitrogen and oxygen together are heated to a temperature of about 3000°C, the nitric oxide gas is formed.
N2 + O2 + heat → 2NO(g)
(ii) Decomposition of calcium carbonate into carbon dioxide and calcium oxide when heated to a 1000°C.
CaCO3 + Heat → CaO(s) + CO2(g)
Q12. State the effect of:
(a) An endothermic reaction
(b) An exothermic reaction on the surroundings.
Answer:
(a) Carbon dioxide present in the atmosphere is trapped by infrared radiations, gives
rise to temperature which is exothermic reaction.
(b) The melting of glaciers by global warming.
Q13. What do you observe when?
(a) An acid is added to a basic solution.
(b) Ammonium chloride is dissolved in water.
Answer:
(a) A chemical process in which an acid and a base or an alkali combine to form salt and water. Salt + Water + Acid + Base
(b) An endothermic reaction occurs when ammonium chloride dissolves in water, and heat is involved Energy is taken in.



