Students of ICSE Class 10 should refer to Nitric Acid ICSE Class 10 Chemistry Questions below which have come in past board exams. You should always go through questions that have come in previous years. This will help you to understand the pattern of questions in ICSE Class 10 Chemistry and prepare accordingly. This will help you to get better marks in ICSE Class 10 Board Exams
ICSE Class 10 Chemistry Important Questions Nitric Acid
Nitric Acid is an important chapter in ICSE Class 10 Chemistry. Our faculty has prepared the following ICSE Class 10 Chemistry Questions and answers based on the latest syllabus and books issued for the current academic year. Please refer to the solved questions below and also see links provided for other chapters.
Nitric Acid ICSE Class 10 Chemistry Questions
Nitric Acid ICSE Class 10 Chemistry Questions
Introduction
• Molecular Formula of Nitric acid – HNO3
• Molecular mass = 1 + 14 + 48 = 63
• Vapour density = ½ × molecular weight = ½ × 63 = 31.5
A. Occurrence of nitric acid
1. What is nitric acid also known as?
Ans. Aqua fortis (strong water).
2. How is Nitric acid formed naturally?
Ans. Occurrence : Found in traces in rain water in the Free State.
i) During Thunder, Lightening, N2 of the air comes in contact with O2 and forms nitric oxide.
N2 + O2 → 2NO (nitric oxide)
ii) Nitric oxide combines with more O2 to form NO2.
2NO + O2 → 2NO2
iii) NO2 combines with rain water to form a very dilute solution of nitric acid & nitrous acid.
2NO2 + H2O → HNO2 + HNO3 OR
4NO2 + O2 + 2H2O → 4HNO3
B. Preparation of nitric acid
3. Answer the following questions with respect to preparation of nitric acid in the laboratory

(a) How is nitric acid prepared in the laboratory?
Ans. It is prepared by the action of a non volatile acid (H2SO4) on the salt of a volatile acid (NaNO3 or KNO3) in distillation flask.
(b) Give the equation of the reaction

Ans. When the temperature is below 200ºC NaHSO4 (Sodium Bisulphate) is formed. Above 200ºC sodium sulphate is formed which is an undesirable product.
i) It Sticks to the glass and is difficult to remove & thus damages the apparatus.
ii) Na2SO4 is a poor conductor of heat and therefore fuel is wasted and apparatus may tend up crack.
iii) At high temperature nitric acid decomposes to give NO2 which gives a brown colouration to the acid.
(d) Why is all glass apparatus used in the laboratory preparation on nitric acid?
Ans. Nitric acid vapours are highly corrosive in nature and may corrode rubber cork, but does not affect glass, hence the apparatus used in laboratory preparation is all glass apparatus.
(e) Why is conc. HCl not used in the preparation instead of conc. H2SO4?
Ans. Conc. HCl is a volatile acid & cannot displace another volatile acid from its compound, conc. H2SO4 is non – volatile and can displace a volatile acid from its compound.
(f) Why is conc. H2SO4 used in the preparation of nitric acid?
Ans. Conc. H2SO4 is a non – volatile acid and can displace volatile acids like HNO3 and HCl from their compounds like KNO3, NaNO3, KCl & NaCl.
(g) How is nitric acid collected in the laboratory?
Ans. Nitirc acid vapours are condensed and collected in the water – cooled receiver.
(h) What precautions are to be taken during the preparation?
Ans. The precautions to be taken are :-
(i) The complete apparatus should be made of glass
(ii) Conc. H2SO4 [non-volatile acid] is used.
(iii) Temperature is maintained around 200ºC.
(i) State the method of identification of the nitric acid formed
Ans. The liquid obtained is heated alone or with copper turnings which evolve reddish brown fumes of Nitrogen dioxide, which turns acidified ferrous sulphate solution brown.
(j) Why is nitric acid obtained brown in colour?
Ans. Pure nitric acid is colourless. Nitric acid obtained in the lab is brown due to the decomposition of the acid during the preparation, the NO2 imparts brown colour to the acid.
4HNO3 → 2H2O + 4NO2 + O2.
(k) How will you remove the brown colour from the acid?
Ans. a) Diluting the acid with water. It will dissolve the NO2.
b) By passing hot air or CO2. This will drive away the NO2 from the acid & oxidize it to nitric acid which is colourless.
4. State the colour of – (i) pure nitric acid (ii) nitric acid obtained in the laboratory (iii) nitric acid obtained in the laboratory after passage of air or addition of water to it.
Ans. (i) Colourless (ii) Yellowish brown (iii) Colourless
5. Answer the following questions with respect to industrial preparation of nitric acid.
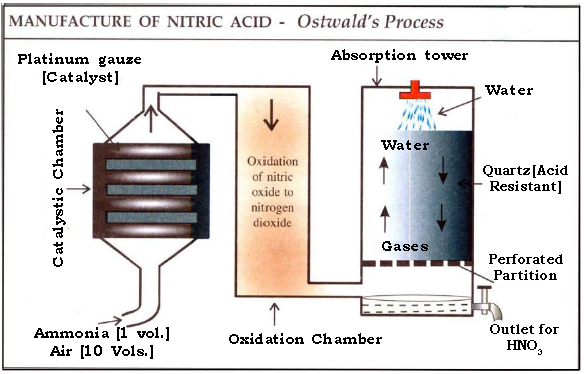
(a) Name the method used to prepare nitric acid on a large scale by catalytic oxidation of ammonia.
Ans. Ostwald’s process.
(b) Give the equations of reactions for the preparation.
Ans. i. Catalytic chamber

(c) Why is the tower packed with quartz?
Ans. Tower is packed with quartz since it is acid resistant and it is packed in layers to slow down the movement of nitrogen dioxide and initiating better solvation in water sprinkled from above the tower.
(d) How is the temperature maintained in Ostwald’s process?
Ans. In catalytic chamber the temperature is maintained at 700 – 800ºC. Catalytic oxidation of ammonia is an exothermic process and heating is done initially. As electric heating is stopped temperature stays at 700 – 800ºC due to the heat of the reaction.
(e) Why is higher ratio of the reactant air required in the manufacture of nitric acid?
Ans. Oxygen is required in all three reactions in the manufacture. Since, only about 21% by volume of air is oxygen, a higher percentage of air is required in the manufacture of nitric acid.
(f) Why is the temperature lowered during the conversion of NO to NO2?
Ans. Lowering the temperature facilitates the ease of oxidation of NO to NO2 and also minimizes the chances of decomposition of nitrogen dioxide due to higher temperature.
C. Properties of Nitric Acid
6. Give the physical properties of nitric acid.
Ans. Colour : Pure acid is colorless, commercial acid is yellow brown (presence of dissolved NO2).
Odour : Suffocating and choking odour.
Taste : Sour.
Solubility : Highly soluble in water.
Constant : Boiling mixture : 121ºC with 68 % concentration.
7. What happens when conc. HNO3 falls on the skin ?
Ans. Nitric acid is corrosive. If it falls on the skin it causes blisters. It combines with the protein of the skin and forms xanthoproteic acid. This stains skin yellow.
8. Why should Nitric acid be kept in dark bottles ?
Ans. Nitric acid is unstable even at ordinary temperatures. In the presence of sunlight, it is decomposed to form NO2. This gives a brown colour to the acid.
4HNO3 → 2H2 O + 4NO2 + O2.
9. Why distilling or boiling cannot be used to concentrate nitric acid beyond a certain point? How is it concentrated beyond the point?
Ans. Its aqueous solution forms a constant boiling mixture at 121ºC with 68% concentration. That means it boils without change in its composition. Further concentration is possible by distilling the acid under reduced pressure in the presence of conc. sulphuric acid. The acid obtained is called as fuming nitric acid with 98% concentration.
10. Nitric acid is a monobasic acid. Explain
Ans. Dilute nitric acid is a monobasic acid. One molecule of the acid on ionization produces only one hydrogen (H+) ion. It is the presence of the H+ or H3O+ ion which is responsible for its acidic properties.

11. State the colour change observed on adding dilute nitric acid in the following indicators.
a) Litmus b) Methyl orange
c) Phenolphthalein d) Alkaline Phenolphthalein
Ans. a) Blue litmus turns red
b) Methyl orange turns from orange to pink
c) Phenolphthalein remains colourless
d) Alkaline phenolphthalein turns from pink to colourless
12. Nitric acid is a strong oxidizing agent. Explain.
Ans. Nitric acid is an oxidizing agent. Nitric acid gives out nascent oxygen. This is responsible for its oxidising nature.
2HNO3 → H2O + 2NO2 + [O].
The nascent oxygen oxidizes both metals and non – metals.
13. Give the general equations to show the acidic nature of nitric acid.
Ans. (i) Base + dil. nitric acid → Metal nitrate + water
(metal oxide / hydroxide) (salt)
(ii) Metal carbonate + dil. nitric acid → Metal nitrate + water + carbon
/ bicarbonate (salt) dioxide ↑
(iii) Metal sulphite / + dil. nitric acid → Metal nitrate + water + sulphur
bisulphite (salt) dioxide ↑
14. Give the balanced chemical equation of the following reactions:
(a) Action of dilute nitric acid on following alkalis
(i) Caustic potash (ii) Caustic soda (iii) Ammonium hydroxide.
Ans. (i) KOH + HNO3 → KNO3 + H2O
dil.
(ii) NaOH + HNO3 → NaNO3 + H2O
dil.
(iii) NH4OH + HNO3 → NH4NO3 + H2O
dil.
(b) Action of dilute nitric acid on the following metallic oxides.
(i) Copper oxide (ii) Magnesium oxide
(iii) Copper hydroxide (iv) Sodium oxide
(v) Calcium oxide (vi) Magnesium hydroxide
(vii)Zinc oxide (viii) Lead oxide
Ans. (i) CuO + 2HNO3 → Cu(NO3)2 + H2O
dil.
(ii) MgO + 2HNO3 → Mg(NO3)2 + H2O
dil.
(iii) Cu(OH)2 + 2HNO3 → Cu(NO3)2+ 2H2O
dil.
(iv) Na2O + 2HNO3 → 2NaNO3 + H2O
dil.
(v) CaO + 2HNO3 → Ca(NO3)2 + H2O
dil.
(vi) Mg(OH)2 + 2HNO3 → Mg(NO3)2+ 2H2O
dil.
(vii) ZnO + 2HNO3 → Zn(NO3)2 + H2O
dil.
(viii)PbO + 2HNO3 → Pb(NO3)2 + H2O
dil.
(c) Action of dilute nitric acid on the following carbonates.
(i) Lead carbonate (ii) Zinc carbonate
(iii) Calcium carbonate (iv) Sodium carbonate
(v) Sodium bicarbonate (vi) Calcium bicarbonate
Ans. (i) PbCO3 + 2HNO3 → Pb(NO3)2 + H2O + CO2 ↑
dil.
(ii) ZnCO3 + 2HNO3 → Zn(NO3)2 + H2O + CO2 ↑
dil.
(iii) CaCO3 + 2HNO3 → Ca(NO3)2 + H2O + CO2 ↑
(iv) Na2CO3+ 2HNO3 → 2NaNO3 + H2O + CO2 ↑
dil.
(v) NaHCO3 + HNO3 → NaNO3 + H2O + CO2 ↑
dil.
(vi) Ca(HCO3)2 + 2HNO3 → Ca(NO3)2 + 2H2O + 2CO2 ↑
dil.
(d) Action of concentrated nitric acid on following non – metals
(i) Phosphorus (ii) Sulphur (iii) Carbon
Ans. (i) P + 5HNO3 → H3PO4 + H2O + 5NO2 ↑
conc.
(ii) S + 6HNO3 → H2SO4 + 2H2O + 6NO2 ↑
conc.
(iii) C + 4HNO3 → 2H2O + CO2 ↑+ 4NO2 ↑
conc.
(e) Reaction of cold and dilute nitric acid with two metals to release hydrogen gas.
Ans. Mg + 2HNO3 → Mg(NO3)2 + H2↑
1% dil & cold
Mn + 2HNO3 → Mn(NO3)2 + H2↑
1% dil & cold
(f) Action of cold and dilute nitric acid on the following metals.
(i) Copper (ii) Zinc (iii) Iron
Ans. (i) 3Cu + 8HNO3 → 3Cu(NO3)2 + 4H2O + 2NO↑
dil.
(ii) 3Zn + 8HNO3 → 3Zn(NO3)2 + 4H2O + 2NO↑
dil.
(iii) 3Fe + 8HNO3 → 3Fe(NO3)2 + 4H2O + 2NO↑
dil.
(g) Action of concentrated nitric acid on following metals.
(i) Copper (ii) Zinc (iii) Iron
Ans. (i) Cu + 4HNO3 → Cu(NO3)2 + 2H2O + 2NO2↑
conc.
(ii) Zn + 4HNO3 → Zn(NO3)2 + 2H2O + 2NO2↑
conc.
(iii) Fe + 6HNO3 → Fe(NO3)3 + 3H2O + 3NO2↑
conc.
(h) Oxidation of following inorganic compounds using nitric acid.
(i) Hydrogen sulphide
(ii) Sulphur dioxide
(iii) Ferrous sulphate
Ans. (i) 3H2S + 2HNO3 → 3S + 4H2O + 2NO↑
dil.
(ii) 3SO2 + 2HNO3 + 2H2O → 3H2SO4 + 2NO↑
dil.
(iii) 6FeSO4(aq) + 3H2SO4(aq) + 2HNO3(aq) → 3Fe2(SO4)3 + 4H2O + 2NO↑
dil.
(i) Oxidation of saw dust
Ans. [C6H10O5]n + Hot conc. nitric acid → H2O + CO2 + NO2↑
(j) Nitration of Toluene
Ans. C7H8 + 3HNO3 → C7H5(NO2)3 + 3H2O
hot.conc.
15. Dilute nitric acid shows typical acid base reaction except for the reaction with metals. Why?
Ans. Nitric acid is a strong oxidising agent and the nascent oxygen formed oxidises the hydrogen to water.
16. What is passivity?
Ans. Passivity or inertness is exhibited by certian metals (Aluminium, nickel, chromium) under conditions in which chemical activity is expected.
17. What does passivity of iron mean?
Ans. Iron is rendered passive due to formation of a thin oxide coating on the surface of the metal which prevents further reaction.
18. What is aqua regia?(Royal water)
Ans. This is a solvent of gold & platinum, it is made up of 3 parts of conc. HCl & 1 part of conc. HNO3. Nascent chlorine is liberated. This reacts with gold & platinum to form soluble gold and platinum chloride.
3HCl + HNO3 → 2H2O + NOCl + 2[Cl]
Conc. Conc. (nitrosyl chloride) (nascent chlorine)
Pt + 4[Cl] → PtCl4
Au + 3[Cl] → AuCl3
D. Tests for Nitric Acid
Give the tests for nitric acid.
Ans. 1) Reaction with copper

3) Brown Ring Test for detection of Nitrate Radical:
i) Take dilute HNO3 at room temperature.
ii) Add freshly prepared saturated ferrous sulphate solution.
iii) Then add conc. H2SO4 from the sides of the test tube slowly.
iv) A brown ring of nitroso ferrous sulphate is obtained at the junction of the two liquids.

20. Answer the following questions with respect to Brown ring test.
(a) Give the equation for formation of brown ring.
Ans. 6FeSO4 + Conc. 3H2SO4 + dil 2HNO3 → 3Fe2(SO4)3 + 4H2O + 2NO
FeSO4 + NO → FeSO4.NO
(b) Why is freshly prepared ferrous sulphate solution used ?
Ans. Ferrous sulphate on exposure to air gets oxidized to ferric sulphate. This does not give the brown ring test.
(c) What is the composition of the brown ring?
Ans. Nitrosoferrous Sulphate FeSO4. NO.
(d) The test tube must not be disturbed during the test, why?
Ans. This is because the heat evolved due to the mixing of conc. sulphuric acid assists the decomposition of the unstable brown ring.
E. Uses of Nitric acid
21. Give the uses of nitric acid :
Ans. i) Preparation of aqua regia to dissolve gold and platinum.
ii) To prepare fertilizers like Ca(NO3)2, NH4NO3 and nitro chalk.
(NH4NO3 + CaCO3)
iii) To purify Au Pt & Ag by dissolving the impurities present in it like Cu, Zn etc.
iv) To etch designs on brassware and noble metals.
v) Used as a rocket fuel oxidant.
vi) To prepare explosives like T.N.T.(Trinitrotoluene), KNO3 – Gun powder,
NH4NO3 – fertilizer, AgNO3 – photography.
ADDITIONAL QUESTIONS
I. Name the followings : –
1. Another name for nitric acid.
Ans. Aqua fortis.
2. The undesirable compound formed when conc. H2SO4 is heated with NaNO3 above 200ºC.
Ans. Sodium sulphate.
3. The gas giving nitric acid a brown colour.
Ans. Nitrogen dioxide.
4. The compound formed which causes yellowing of skin when HNO3 falls on it.
Ans. Xanthoproteic acid.
5. A colourless neutral oxide of nitrogen.
Ans. Nitric oxide.
6. The temperature at which conc. HNO3 forms a constant boiling mixture.
Ans. 121ºC.
7. The gas evolved when hot and conc. HNO3 reacts with Cu/Zn.
Ans. Nitrogen dioxide.
8. The gas evolved when cold and dilute HNO3 reacts with Cu/Zn.
Ans. Nitric oxide or nitrogen monoxide.
9. The compound which forms the brown ring.
Ans. Nitrosoferrous sulphate (FeSO4.NO) (brown compound).
10. A metal which turns passive with nitric acid
Ans. Iron/Aluminium.
11. A nitrate of a metal which does not give nitrogen dioxide on heating
Ans. Sodium nitrate or potassium nitrate.
12. A nitrate which on heating leaves no residue behind.
Ans. Ammonium nitrate.
13. Two metals which react with very cold and dilute nitric acid to liberate hydrogen gas.
Ans. Magnesium and Manganese.
14. A non – volatile acid prepared using a volatile acid
Ans. Sulphuric acid.
15. 98% concentrated nitric acid.
Ans. Fuming nitric acid.
II. Fill in the blanks:
1. Aqua regia is a mixture of 3 parts of Conc. _________ (HCl/H2SO4/HNO3) and 1 part of Conc._______ (HCl/H2SO4/HNO3)
2. The catalytic oxidation of ammonia to nitric oxide is a ________ (exothermic/endothermic) process.
3. Magnesium gives _______ (O2, H2, NO) gas with very cold and dilute nitric acid.
4. ___________ (Iron / Copper) becomes passive in concentrated nitric acid.
5. The oxidised product obtained on reaction of H2S gas with dil. HNO3 is __________ (SO2/ H2SO4/ S)
6. A mineral acid obtained from conc. nitric acid on reaction with non – metal is ________ (hydrochloric acid / phosphoric acid / Carbonic acid)
7. The reaction of ________ ( calcium carbonate / calcium oxide / calcium sulphite) with dilute nitric acid liberates only water as a byproduct.
8. Brown ring test is used to detect __________ (nitrate/sulphate/carbonate) radical.
III. Correct the following sentences:
1. HNO3 is a strong reducing agent
Ans. HNO3 is a strong oxidising agent.
2. NaNO3 gives NO2 and O2 on heating
Ans. NaNO3 gives NaNO2 and O2 on heating
3. Sodium sulphite reacts with dil HNO3 giving NO2 gas.
Ans. Sodium sulphite reacts with dil HNO3 giving SO2 gas.
IV. Answer the following
1. State your observations and give equations for action of heat on following nitrates.
(a) Potassium Nitrate (KNO3) :
Ans. The white crystals melt, a colourless, odourless, neutral gas is evolved which relights a glowing splinter. The residue fuses with the glass.
2KNO3 → 2KNO2 + O2 ↑
(b) Sodium Nitrate (NaNO3) :
Ans. The white crystals melt, a colourless, odourless, neutral gas is evolved which relights a glowing splinter. The residue fuses with the glass.
2NaNO3 → 2NaNO2 + O2 ↑
(c) Zinc Nitrate :
Ans. These white crystals on heating, leave behind a residue which is yellow, when hot & white when cold.
A reddish brown acidic gas which has a pungent odour is evolved.
2Zn(NO3)2 → 2ZnO + 4NO2 + O2
(d) Lead Nitrate :
Ans. The residue left behind is yellow, in colour (PbO – lead monoxide). A reddish brown acidic gas which has a pungent odour is evolved.
2Pb(NO3)2 → 2PbO + 4NO2 + O2
Yellow – Hot
(e) Copper Nitrate :
Ans. Blue coloured crystals which on heating leaves behind a black residue of CuO. Reddish brown acidic gas which has a pungent odour is evolved.
2Cu(NO3)2 → 2CuO + 4NO2 + O2
(f) Mercuric nitrate :
Ans. Reddish brown gas is evolved and a greenish silvery mirror residue is left behind
Hg(NO3)2 → Hg + 2NO2 + O2
(g) Silver Nitrate :
Ans. Reddish brown fumes are evolved and a residue which is silver grey in colour left behind.
2Ag NO3 → 2Ag + 2NO2 + O2
2. Select the letters A, B, C, D or E, which form the gaseous products of the reactions from 1 to 5.
A: Nitrogen dioxide only B: Nitric oxide only
C: Hydrogen D: Nitrogen dioxide and Oxygen.
E: Nitrogen dioxide with carbon dioxide
1. Reaction of manganese with cold very dil. nitric acid.
2. Reaction of sulphur with conc. nitric acid.
3. Reaction of zinc with dil. nitric acid.
4. Reaction of carbon with conc. nitric acid.
5. Heat on nitric acid.
Ans. 1. C: Hydrogen
2. A: Nitrogen dioxide only
3. B: nitric oxide only
4. E: Nitrogen dioxide with carbon dioxide
5. D: Nitrogen dioxide and oxygen
3. Why is NO2 called a mixed acid anhydride ?
Ans. NO2 combine with water to form 2 acids – nitrous acid & nitric acid. Thus it is called a mixed acid anhydride.
2NO2 + H2O → HNO3 + HNO2
4. What is Nitration Reaction?
Ans. Organic compounds like benzene, toluene, phenol, etc. undergo nitration reaction that is one or more hydrogen atoms are substituted or replaced by the nitro group – NO2 from the organic compound.
5. What is fuming Nitric acid?
Ans. Conc nitric acid is highly hydroscopic and decomposes to nitrogen dioxide, oxygen & water thus fumes in air hence named fuming nitric acid. It is more powerful oxidising agent than 68% conc. nitric acid.
6. State why nitric acid i) stains the skin (ii) cannot be concentrated beyond 68% by boiling.
Ans. (i) Nitric acid reacts with proteins of skin forming xanthoproteic acid (yellow compound) which stains the skin yellow.
(ii) Nitric acid forms a constant boiling mixture. Which boils without change in its composition.
7. Name the oxidised product when the following ‘1 to 5’ react with nitric acid.
1. Sulphur [with conc. acid]
2. Zinc. [with dilute acid]
3. Aqueous solution of SO2 [with dilute acid]
4. Acidified iron (II) Sulphate [with dilute acid]
5. Carbon [with conc. acid]
Ans. 1. Sulphuric acid
2. Zinc nitrate
3. Sulphuric acid
4. Iron (III) sulphate
5. Carbon dioxide
V. Balanced chemical equations : –
1. Give blanaced equations for the following conversion A to E.

Ans. 1. A : Cu + 2HNO3 → Cu(NO3)2 + H2O + NO2
conc.
OR
3Cu + 8HNO3 → 3Cu (NO3)2 + 4H2O + 2NO
dil.
B : 2Cu(NO3)2 → 2CuO + O2 + 4NO2↑
C : 2CuO + C → 2Cu + CO2↑
2. D: S + 6HNO3 → H2SO4 + 2H2O + 6NO2↑
E : 3SO2 + 2H2O + 2HNO3 → 3H2SO4 + 2NO ↑ OR
2SO2 + 2H2O + O3 → 2H2SO4
2. Complete the following chemical equation & balance them.
1. MgO + HNO3 →
Ans. MgO + 2HNO3 → Mg (NO3 )2 + H2O
2. CaO + HNO3 →
Ans. CaO + 2HNO3 → Ca(NO3)2 + H2O
3. KOH + HNO3 →
Ans. KOH + HNO3 → KNO3 + H2O
4. PbCO3 + HNO3 →
Ans. PbCO3 + 2HNO3 → Pb(NO3) 2+ H2O + CO2
5. NaHCO3 + HNO3 →
Ans. NaHCO3 + HNO3 → NaNO3 + H2O + CO2
6. Cu + HNO3 →
conc.
Ans. Cu + 4HNO3 → Cu(NO3)2 + 2H2O + 2NO2
7. H2S + HNO3 →
dil.
Ans. H2S + 2HNO3 → 3S + 4H2O + 2NO
8. C + HNO3 →
conc.
Ans. C + 4HNO3 → 2H2O + CO2 + 4NO2
9. Mn + HNO3 →
1% dil & cold.
Ans. Mn + 2HNO3 → Mn(NO3)2 + H2
10. 3Zn + HNO3 →
dil.
Ans. 3Zn + 8HNO3 → 3Zn(NO3)2 + 4H2O + 2NO



