Students of ICSE Class 10 should refer to Sulphuric Acid ICSE Class 10 Chemistry Questions below which have come in past board exams. You should always go through questions that have come in previous years. This will help you to understand the pattern of questions in ICSE Class 10 Chemistry and prepare accordingly. This will help you to get better marks in ICSE Class 10 Board Exams
ICSE Class 10 Chemistry Important Questions Sulphuric Acid
Sulphuric Acid is an important chapter in ICSE Class 10 Chemistry. Our faculty has prepared the following ICSE Class 10 Chemistry Questions and answers based on the latest syllabus and books issued for the current academic year. Please refer to the solved questions below and also see links provided for other chapters.
Sulphuric Acid ICSE Class 10 Chemistry Questions
Sulphuric Acid ICSE Class 10 Chemistry Questions
Introduction
Sulphuric Acid (H2SO4)
Relative Molecular Mass = 2 × 1 + 32 + 16 × 4 = 98
Vapour Density = 98/2 = 49
Sulphuric acid is the King of Chemicals.
Occurence :
In Free State – Minute traces in hot springs, near sulphide beds.
In Combined State – Metal sulphates such as barium sulphate, calcium
sulphate, magnesium sulphate etc.
A. Preparation of Sulphuric acid
1. State why sulphuric acid is referred to as : a) King of chemicals b) oil of vitriol
Ans. a) Sulphuric acid is used on a large scale in a number of industries, hence is referred to as King of chemicals.
b) Sulphuric acid was initally obtained as an oily viscous liquid by heating crystals of green vitriol, hence was referred to as oil of vitriol.
2. Give balanced chemical equation for the reaction to convert the following compounds to sulphuric acid in a single step:
a) Sulphur b) Chlorine c) Sulphur trioxide d) Sulphuryl chloride
Ans. a) S + 6HNO3 → H2SO4 + 2H2O + 6NO2
conc.
b) 2H2O( ℓ ) + SO2(g) + Cl2(g) → 2HCl(aq) + H2SO4(aq) water sulphur chlorine Hydrochloric sulphuric
dioxide acid acid
c) SO3 + H2O → H2SO4
d) SO2Cl2 + 2H2O → 2HCl + H2SO4
Sulphuryl
chloride
B. Manufacture of Sulphuric acid
3. Answer the following questions with respect to manufacture of sulphuric acid.

a) State the purpose of contact process
Ans. Contact process is used to manufacture sulphuric acid on a large scale.
b) Give the equation for the conversion of i) Sulphur and ii) Iron pyrites to sulphur dioxide in the first step of the contact process.
Ans. i) S + O2→ SO2↑
ii) 4FeS2 + 11O2 → 2Fe2O3 + 8SO2 ↑
c) Why is burning of iron pyrites in oxygen preferred to burning iron pyrites in purified air?
Ans. Burning of iron pyrites in oxygen is preferred to purified air since heat energy is wasted in heating the unreactive nitrogen component present in air.
d) Why is sulphur dioxide gas purified before entering contact tower?
Ans. Sulphur dioxide produced contains impurities like dust – particles or pyrite dust and arsenious oxide. These impurities poison or deactivate the catalyst, thereby reducing its efficiency.
e) How is dust and moisture removed from sulphur dioxide during purification?
Ans. Dust particles are removed by passing sulphur dioxide through dusting and scrubbing tower. Moisture is removed by passing the gas through conc. sulphuric acid in the drying tower.
f) How are the arsenious impurities removed? Why is it done?
Ans. The arsenious impurities are removed by passing the gas over ferric hydroxide. They are removed as these impurities tend to deactivate the catalyst (poison the catalyst) thereby reducing its efficiency.
g) State the conditions required in the catalytic oxidation of sulphur dioxide to sulphur trioxide in the Contact tower with respect to the following:
i) Catalyst ii) Promoter iii) Temperature and iv) Pressure.
Ans. i) Catalyst: Vanadium pentoxide
ii) Promoter: Potassium oxide
iii) Temperature: 450oC – 500oC
iv) Pressure: 1- 2 atm
h) Why is vanadium pentoxide a better catalyst than platinum?
Ans. Vanadium pentoxide is preferred to Pt as :
• It is cheaper
• less easily poisoned by impurities.
i) Why is the catalyst only initially heated?
Ans. Catalyst used is only initially heated, since the catalytic oxidation of sulphur dioxide is an exothermic reaction and the heat produced during reaction maintains the required temperature [450ºC – 500ºC].
j) Give the balanced chemical equation of the reaction taking place in the contact tower.
Ans.
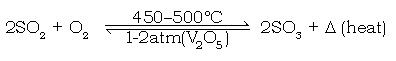
k) State why an optimum temperature of 450oC – 500oC maintained in the above catalytic oxidation.
Ans. The catalytic oxidation of sulphur dioxide is an exothermic reaction, which is favoured by lowering the temperature. However, if the temperature is too low, the reaction tends to slow down. Hence, an optimum temperature
of 4500C – 5000C is maintained.
l) How is sulphuric acid obtained?
Ans. The vapours are first dissolved in sulphuric acid to give oleum and then calculated amount of water is added to give the desired concentration of sulphuric acid.
SO3 + H2SO4 → H2S2O7
(Oleum)
Dilution :
H2S2O7 + H2O → 2H2SO4
m) Why is sulphur trioxide not directly dissolved in water?
Ans. Although SO2 is an acid anhydride of sulphuric acid, it is not directly absorbed in water as the reaction is highly exothermic resulting in the formation of a dense fog, which is difficult to condense.
C. Properties of Sulphuric acid
4. Give the physical properties of Sulphuric acid.
Ans. (i) It is an oily, dense, colourless and odourless liquid.
(ii) Sulphuric acid has a relative density of 1.84 at 15ºC.
(iii) Sulphuric acid freezes at 10.4ºC, to colorless crystals and boils at 338°C.
(iv) Sulphuric acid is soluble in water in all proportions.
(v) Pure sulphuric acid is almost a non-conductor of electricity but dilute acid becomes a very good conductor and behaves like a strong electrolyte.
(vi) Forms a constant boiling mixture at 3380C containing 98.5% acid.
5. Give reason why concentrated sulphuric acid is kept in air tight bottles.
Ans. Concentrated sulphuric acid being hygroscopic, it absorbs moisture from the atmosphere and becomes dilute, hence concentrated sulphuric acid is kept in air tight bottles.
6. How can concentrated sulphuric acid be diluted? Why?
Ans. Acid should be diluted by adding small quantities of acid into large quantity of water. This is because a large amount of heat is given out during dissolution as this reaction is exothermic. So if water is added to the acid,
water being lighter may float on the acid and the heat generated may cause the acid to spurt on the person causing severe burns. When acid is added to water in small quantities with constant stirring, then it being heavier sinks to the bottom and the heat evolved is distributed in all directions. And no spurting will occur.

7. Why can’t sulphuric acid be concentrated beyond 98%?
Ans. The acid of this concentration forms a constant boiling mixture with the remaining small quantity of water at 338ºC. Hence sulphuric acid cannot be concentrated beyond 98.33% by boiling.
8. Give reason why sulphuric acid
i) behaves as an acid when dilute
ii) is dibasic in nature
iii) acts as a dehydrating agent
Ans. i) Pure acid is not acidic in nature as pure acid does not dissociate producing H+ ions, which inturn does not produce H3O+ ions which gives acidic nature. But upon dilution, acid dissociates producing H+ ions, which inturn produces H3O+ ions. Hence acid when dilute behaves as an acid.
ii) One molecule of sulphuric acid ionizes almost completely in water to produce 2 hydrogen ions as follows :
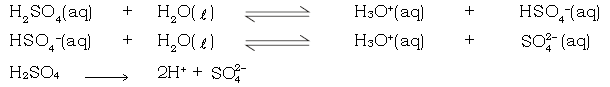
These two hydrogen ions combine with two hydroxyl ions of a base to produce 2 molecules of water.
iii) Concentrated sulphuric acid has great affinity for chemically combined
water molecules and hence is used as a dehydrating agent.
9. Show the effect of the sulphuric acid on the following indicators :
Ans. No. Indicators Effect Of sulphuric Acid
1. Blue Litmus Blue Litmus turns red.
2. Methyl Orange It turns from orange to pink.
3. Phenolphthalein Phenolphthalein remains colourless.
4. Alkaline Phenolphthalein It turns from pink to colourless
10. Convert dil. H2SO4 to –
i) Hydrogen ii) Carbon dioxide iii) Sulphur dioxide
iv) Hydrogen sulphide v) An acid salt vi) A normal salt.
Ans. i) (a) Mg + H2SO4 → MgSO4 + H2 ↑
(b) Zn + H2SO4 → ZnSO4 + H2 ↑
(c) F e + H2SO4 → FeSO4+ H2 ↑
ii) (a) Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2 ↑
(b) 2NaHCO3 + H2SO4 → Na2SO4 + 2H2O + 2CO2 ↑
(c) MgCO3 + H2SO4 → MgSO4 + H2O + CO2 ↑
(d) ZnCO3(s) + H2SO4(aq) → ZnSO4(aq) + H2O + CO2 ↑
(e) 2KHCO3(aq) + H2SO4(aq) → K2SO4(aq) + 2H2O( ↑ )+ 2CO2 ↑
iii) (a) Na2SO3 + H2SO4 → Na2SO4 + H2O + SO2 ↑
(b) 2 NaHSO3 + H2SO4 → Na2SO4 + 2 H2O + SO2 ↑

11. Give equations of conc. H2SO4 giving the oxidised products –
(i) Carbon dioxide (ii) Sulphur dioxide
(iii) Phosphoric acid (iv) Copper (II) sulphate
(v) Iodine (vi) Sulphur
(vii) Bromine
Ans. i) C + 2 H2SO4 → CO2(g) + 2 H2O + 2 SO2 →
ii) S + 2 H2SO4 → 3 SO2 ↑ + 2 H2O
iii) 2 P + 5 H2SO4 → 2 H3PO4 + 2 H2O + 5 SO2 ↑
iv) Cu + 2 H2SO4 → CuSO4 + 2 H2O + SO2 ↑
v) 2 HI + H2SO4 → I2 + 2 H2O + SO2 ↑
vi) H2S + H2SO4 → S + 2 H2O + SO2 ↑
vii) 2 HBr + H2SO4 → Br2 + 2 H2O + SO2 ↑
12. Give the equation for the action of concentrated sulphuric acid on the following
i) Glucose
ii) Cellulose
iii) Sucrose
iv) An organic acid containing one carbon atom and two hydrogen atoms or Formic acid.
v) An organic acid containing two carbon atoms and two hydrogen atoms or oxidic acid.
vi) An alcohol.
vii) Hydrated copper (II) sulphate.
Ans.


13. State the property involved and give the equations for formation of two different acids from conc. H2SO4.
Ans. Sulphuric acid is a non volatile acid that is it has a high boiling point as compared to hydrochloric and nitric acid. Hence on heating with salts of more volatile acids, conc. H2SO4 displaces the acids from their salts. Thus non volatile acid is used to displace a volatile acid from its salt.

D. Test for Sulphuric acid
14. State how addition of the following compounds serves as a test for conc. H2SO4
(i) Copper (ii) NaCl
Ans. (i) Action of heat on Copper & conc. H2SO4 gives sulphur dioxide [SO2]

Metal copper reacts with conc. sulphuric acid to give SO2 gas which decolourises potassium permanaganate solution.
(ii) Action of heat on sodium chloride & conc. H2SO4 gives hydrogen chloride [HCl]
When added to NaCl it gives fumes which are colourless and when a glass rod is dipped in ammonium hydroxide solution is brought near it, dense white fumes are seen, indicating that the gas is HCl.
15. State how addition of the following compounds serves as a test for dil. H2SO4
(i) BaCl2 (ii) Pb(NO3)2
Ans. (i) The acid gives a white ppt of BaSO4 when BaCl2 is added to it.
BaCl2(aq) + H2SO4(aq) → 2HCl(aq) + BaSO4 ↑
Barium Sulphuric Hydrochloric Barium sulphate
chloride acid acid (white)
(ii) A white ppt of PbSO4 is formed when it is added to lead nitrate solution.
Pb(NO3)2(aq) + H2SO4(aq) → 2HNO3(aq) + PbSO4 ↑
Lead nitrate sulphuric Nitric Lead sulphate
acid acid (white)
E. Uses of sulphuric acid
1. Fertilizers – manufacturing process of fertilizers such as ammonium sulphate, super phosphate of lime etc.
2. Chemical industry – manufacturing of hydrochloric acid, nitric acid, phosphoric acid, metal sulphates.
3. Explosive industry – nitroglycerine and tri – nitro toluene (T.N.T.).
4. Petroleum industry – to remove sulphur and other compounds
5. In metallurgy – extraction of copper, purification of gold, pickling of metals
6. In laboratory – a drying agent and as a dehydrating agent
7. In storage batteries – lead storage batteries used in car accumulators.
ADDITIONAL QUESTIONS
I. Name the following :
(a) The volatile acid than can be used to prepare H2SO4.
Ans. Conc. Nitric acid.
(b) Catalyst used in contact process.
Ans. Vanadium pentoxide.
(c) Basicity of H2SO4.
Ans. Dibasic / two.
(d) The salt that can give H2S gas on reaction with dil H2SO4.
Ans. Sulphide Salt.
(f) A neutral oxide of carbon formed when H2SO4 (conc.) reacts with formic acid.
Ans. Carbon monoxide.
(g) An organic acid which reacts with conc. H2SO4 forms 2 oxides of C i.e CO and CO2.
Ans. Oxalic acid.
(h) Black substance formed when sugar is burnt strongly.
Ans. Carbon.
(i) The metal that can react with conc. H2SO4
Ans. Copper.
(j) Number of acid salts H2SO4 has.
Ans. One (NaHSO4).
(k) Gas obtained by dehydration of ethanol.
Ans. Ethene.
(l) Gas with rotten egg smell.
Ans. Hydrogen sulphide.
(m) Drying agent for SO2 gas.
Ans. Conc. Sulphuric acid.
(n) Two metals that react vigorously and explosively with dil. H2SO4 acid.
Ans. Sodium and potassium.
(o) Name the chemical used to dissolve SO3 and the product formed in contact process.
Ans. Conc. sulphuric acid; Oleum.
II. Match the conversions in column ‘X’ using sulphuric acid, with the type of chemical property of sulphuric acid it represents in column ‘Y’
A B
1. Nitre → Nitric acid a. As an oxidising agent
2. Copper [II] oxide → copper [II] sulphate b. As a dibasic acid
3. Copper → Copper [II] sulphate c. As an acid when dilute
4. Ethanol → Ethene d. As a least or non – volatile acid
5. Sodium hydroxide → Sodium bisulphate e . As a dehydrating agent
Ans. 1. – As a least or non – volatile acid
2. – As an acid when dilute
3. – As an oxidising agent
4. – As a dehydrating agent
5. – As a dibasic acid
III. Select the correct substance from the substances A to J which react with sulphuric acid to give the product 1 to 10. [State whether the acid used in each case is dilute or concentrated].
A : Iron B : Sodium carbonate C: Sodium chloride D: Formic acid E: Sodium nitrate F: Sodium sulphite G: Ethyl alcohol b: Sodium sulphide I: Sodium hydroxide [excess] J: Hydrogen sulphide
1. Product – Sulphur dioxide 2. Product – Sulphur
3. Product – Hydrogen 4. Product – Hydrochloric acid
5. Product – Sodium sulphate 6. Product – Carbon dioxide
7. Product – Carbon monoxide 8. Product – Nitric acid
9. Product – Hydrogen sulphide 10. Product – Ethene
Ans. A: Iron – 3. Hydrogen. Dilute H2SO4
B: Sodium carbonate – 6. Carbon dioxide. Dilute H2SO4
C: Sodium chloride – 4. Hydrochloric acid. Concentrated H2SO4
D: Formic acid – 7. Carbon monoxide. Concentrated H2SO4
E: Sodium nitrate – 8. Nitric acid. Concentrated H2SO4
F: Sodium sulphite – 1. Sulphur dioxide. Dilute H2SO4
G. Elthyl alcohol – 10. Ethene. Concentrated H2SO4
H: Sodium sulphide – 9. Hydrogen sulphide. Dilute H2SO4
I: Sodium hydroxide – 5. Sodium sulphate. Dilute H2SO4
J: Hydrogen sulphide – 2. Sulphur. Concentrated H2SO4
IV. Select the correct answer from the choice in brackets.
1. The oxidised product obtained when sulphur reacts with conc. H2SO4.
[H2S/SO2/H2SO3].
2. The dehydrated product obtained when cane sugar reacts with conc.
H2SO4. [CO/C/CO2]
3. The type of salt formed when excess of caustic soda reacts with sulphuric acid. [acid salt/normal salt].
4. The oxidised product obtained when hydrogen sulphide reacts with conc.
H2SO4. [SO2/S/H2O].
5. The salt which reacts with dil. H2SO4 acid to give an insoluble ppt.
[Cu(NO3)2/ Zn(NO3)2/Pb(NO3]2.
Ans. 1. SO2
2. C
3. normal salt
4. S
5. Pb(NO3)2
V. State the role played by sulphuric acid in the following reactions.
1. Conc. Sulphuric acid reacts with NaCl to prepare HCl.
Ans. Non – volatile acid.
2. Conc. Sulphuric acid reacts with formic acid to form CO.
Ans. Dehydrating agent.
3. Active metal reacts with dil. sulphuric acid to displace / liberate hydrogen.
Ans. Acid behaviour.
4. Conc. H2SO4 used in washing bottles in preparation of HCl.
Ans. Drying agent.
5. Zinc reacts with conc. H2SO4 to release SO2.
Ans. Oxidising agent.
6. Blue copper sulphate crystals turn white when conc. H2SO4 is added.
Ans. Dehydrating agent.
VI. Chemical equations.
a) Write balanced equations for.
(1) Roasting of Iron pyrites.

(2) Magnesium and dil sulphuric acid.
Ans. Mg + H2SO4 → MgSO4 + H2 ↑
(3) Calcium carbonate reacts with dil. sulphuric acid.
Ans. CaCO3 + H2SO4→ CaSO4 + H2O + CO2 ↑
dil.
(4) Conversion of Iron Sulphide to Iron sulphate.
Ans. FeS + H2SO4 → FeSO4 + H2S ↑
dil.
(5) Sulphuric acid reacts with sodium sulphite.
Ans. Na2SO3 + H2SO4 → Na2SO4 + H2O + SO2 ↑
dil.
(6) Sulphuric acid reacts with sodium nitrate
Ans
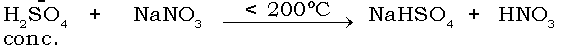
(7) Carbon reacts with conc. sulphuric acid.
Ans. C + 2H2SO4 → CO2 + 2H2O + 2SO2 ↑
conc.
(8) Hydrogen Iodide reacts with conc sulphuric acid.
Ans. 2HI + H2SO4 → I2 + 2H2O + 2SO2 ↑
conc.
(9) Oxalic acid reacts conc. H2SO4
Ans.
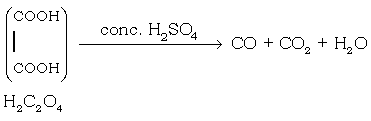
b) Write equation to show the conversions A, B, C, D, E, F and G.


VII. State your observations when.
a. Iron (II) sulphide reacts with dil H2SO4.
Ans. • Black compound dissolves
• Light green solution formed
• Gas with rotten egg smell is given out
b. Mg is reacted with dil H2SO4.
Ans. • Metal dissolves with dil. H2SO4.
• Gas liberated which burns with a pop sound a pale blue flame.
c. Lead nitrate is added to dil H2SO4
Ans. A white ppt is formed which is insoluble in all mineral acids.
d. A beaker of conc. H2SO4 is left open to the atmosphere.
Ans. Volume of the acid increases after sometime.
e. Water is added to conc. H2SO4.
Ans. The water spurts out.
f. FeSO4 crystals come in contact with conc H2SO4
Ans. • Green colour disappears.
• Crystals turn white amorphous.
g. NaCl is added to conc. sulphuric acid
Ans. A colourless gas is liberated which gives dense white fumes when a glass rod dipped in ammonia solution is brought near it.
h. Barium chloride solution is added to dilute sulphuric acid
Ans. A white precipitate is observed.
i. Copper turnings are added to concentrated sulphuric acid
Ans. A colourless gas is liberated, leaving behind a blue coloured solution.
j. Few drops of conc. H2SO4 is added to crystalline copper sulphate or blue vitriol.
Ans. The blue crystalline copper sulphate turns to white amorphous powder.
VIII. Answer the following with respect to the conversion given below.
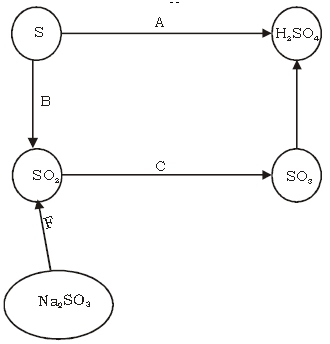
a. Name the catalyst in reaction C.
Ans. Platinum or vanadium pentoxide.
b. Give conversion of SO3 to sulphuric acid by the Industrial method of manufacturing.
Ans. SO3 + H2SO4 → H2S2O7
Oleum
H2SO7 + H2O → 2H2SO4
c. Name the process A.
Ans. Oxidation
d. Write equation of B.
Ans.

e. Write equation for C.
Ans.

f. Write equation for F.
Na2SO3 + H2SO4 → Na2SO4 + H2O + SO2 ↑
IX. Answer the following:
1. State the difference between a drying agent and a dehydrating agent.
Ans. A Drying agent :
• Substances used to remove moisture or wetness from liquids, solids or gases.
• Water removed is physically (externally).
• The drying agent must not react or bring about any chemical change in the substance that is dried.
• E.g. :- Conc. H2SO4, P2O5
A Dehydrating agent :
• Substances that have great affinity for water.
• Water removed is a part of the crystal molecule i.e. Chemically, combined water.
• A dehydrating agent brings about some change in the compound.
E.g. :- Loss of colour (Blue CuSO4 crystals turn white).
E.g. :- Conc. H2SO4
2. State three different chemical compounds other than acids manufactured industrially from sulphuric acid.
The three different compounds are :
Ans. (1) Ammonium sulphate, Superphosphate of lime fertilizers.
(2) Artificial fibres [Nylon, Rayon.]
(3) Explosives [Tri-Nitrotoluene, tri-nitro glycerine.]
3. Give reasons :
a. Sulphuric acid not used to dry hydrogen sulphide gas.
Ans. Sulphuric acid is a strong oxidising agent, it oxidises hydrogen sulphide gas to sulphur and hence is not used to dry it.
b. A piece of wood becomes black when concentrated sulphuric acid is poured on it.
Ans. Sulphuric acid oxidises the cellulose present in the wood to carbon which is black in colour, hence the piece of wood becomes black when concentrated sulphuric acid is poured on it.
c. Sulphuric acid forms two types of salts with an alkali.
Ans. Sulphuric acid is a dibasic acid, it reacts with alkalis to form two types of salts, acid salt and normal salt.
d. Concentrated sulphuric acid acts as a drying agent.
Ans. Concentrated sulphuric acid has a strong affinity towards water and hence acts as a drying agent.
e. The gaseous product obtained differs when zinc reacts with dilute and concentrated sulphuric acid respectively.
ans. Dilute sulphuric acid shows typical acidic property and hence liberates hydrogen gas on reaction with active metal zinc; whereas concentratedsulphuric acid is a strong oxidising agent and hence liberates sulphur dioxide gas on reaction with the metal zinc.
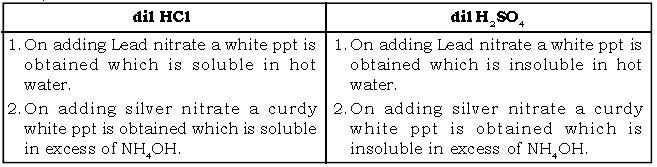
X. How will you distinguish between the following
a. dil HCl & dil H2SO4
Ans.
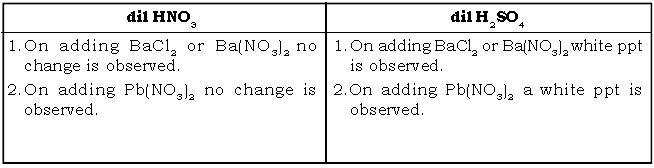
b. dil HNO3 & dil H2SO4
Ans.
c. dil H2SO4 & conc. H2SO4
Ans.




