Students should refer to Heat ICSE Class 10 Physics Board Exam Questions provided below with solutions. These will help the students to understand the type of questions which have been asked in previous year examinations and the type of solutions which the student should give to get good marks. You should also refer to ICSE Class 10 Physics Sample papers for more practice
ICSE Class 10 Physics Heat Important Questions
Students should learn the important questions and answers given below for Chapter Heat in Physics for ICSE Class 10. These board questions are expected to come in the upcoming exams. Students of ICSE Class 10th should go through the Important questions and answers ICSE Class 10 Physics which will help them to get more marks in exams.
Heat ICSE Class 10 Physics Board Exam Questions
Heat ICSE Class 10 Physics Board Exam Questions
1) Why does stone lying in the sun get heated up very much, whereas water lying in the sun for the same duration gets heated very little?
Answer:
Water has the specific heat capacity c= 4,200 J/kg K which is about 5 times the specific heat capacity of the stone. So when the sun shines, water heats up very slowly whereas the stone heats up very fast. That is why the stone gets heated up very much as compared to the water.
2) On a hot summer day, the water in a swimming pool remains comfortably cool. Give reason.
Answer:
On a hot summer day when the temperature is 40 0 C and above, the water in a swimming pool remains comfortably cool (may be around 25 0 C) because of the very high specific heat of water. The rise in temperature (ΔT = Q/mc) is inversely proportional to the specific heat capacity. So the rise in temperature of the water is very small.
3) Why does atmospheric temperature fall sharply after hailstorm?
Answer:
Atmospheric temperature falls sharply after hailstorm because hail is actually ice. Once it reaches the earth’s atmosphere it melts and thus cools the air. Every kilogram of ice on melting absorbs 336000 J of heat atmosphere.
4) Why is aluminium best suited for measurement of the temperature of a furnace?
Answer:
Aluminium is best suited for measurement of the temperature of a furnace because amongst metals aluminium has the highest specific heat capacity (c = 900 J kg -1 K -1 ) due to
which it will transfer more heat than other metals for the same mass and temperature change.
5) Small islands have moderate temperature changes than large land masses in the same latitude. Give reason.
Answer:
Small islands have much more temperate climate (moderate change in temperature) than large land masses in the same latitude. As we know that ΔT ∞ 1/c, temperature variations on land masses are small because of the high specific heat capacity of water. The coastal regions face the effect of continentality. Places near coasts have a small range of temperatures because it faces continentality. Seas and oceans have a moderating influence on the coastal regions. Water has a unique ability to absorb heat and keep it under lock and key. Therefore, hot air over the land moves up and creates a low-pressure region.

6) Why does a location close to water reservoir affect the air temperature?
Answer:
A place close to a large water reservoir is not so hot during summer than those far away from water reservoirs because water due to its large specific heat capacity absorbs a large quantity of heat. Similarly, in winter, the water body cools down and gives off a large quantity of heat and therefore, it is not so cold in winter in such places.
7) Why is wet cloth or pad put on the forehead of a person running a high fever?
Answer:
When a person has a fever, his body temperature becomes more than his normal body temperature. Therefore the doctors recommend putting wet strips of cloth on the forehead of a person with a high fever. This will result in the water to evaporate and absorb the heat from the body resulting in cooling and lowering of the body temperature. Thus, it lowers the temperature of the brain to protect it from any damage due to high temperature.

8) Why do icebergs sometimes reach the equatorial regions?
Answer:
Ice has high specific latent heat of fusion of ice. When large sized glaciers get picked up by the ocean currents, it melts slowly, due to which the icebergs sometimes reach the equatorial regions in the sea.
9) Why there is year long supply of water in snow-fed rivers?
Answer:
In summer, the snow melts slowly on the mountains because every kilogram of snow requires 336000 J of heat energy in order to form water at 0 0 C. Thus, there is year long supply of water in snow-fed rivers.
10) Why do we feel cool after hot water bath?
Answer:
We feel cool after hot water bath because water being at a higher temperature, the rate of evaporation becomes fast, thereby giving a sensation of cooling. So after a bath the water clinging to your skin starts to evaporate. This evaporating water will suck up the heat energy from the droplets and result in cooling down your body.
11) Why do the exposed water pipes burst during a severe frost?
Answer:
Exposed water pipes burst during a severe frost because of expansion of water in the pipes on freezing and this causes an increase in pressure inside the pipe. When the pressure gets too high for the pipe to contain, it ruptures.
12) Why do you generally catch a cold if you put on wet clothes for a long time?
Answer:
If we wear wet clothes for a long time, it may cause overcooling of the body due to rapid evaporation of water. Water, while evaporating, takes latent heat from the body thus, lowers the resistance of the body to cold.
13) Why does heat in summer get very oppressive just after rainfall?
Answer:
Heat in summer gets oppressive (meaning: unbearable) especially just after rainfall because air is almost completely saturated with water vapour. Due to which evaporation of sweat from our body almost stops and thereby body stops losing heat.
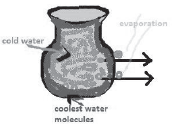
14) Why does the water remain cool in hot summer but not in rainy season in earthen pitchers?
Answer:
In earthen pitchers, water remains cool in hot summer because water oozes out from the pores of pitcher. It evaporates partly taking heat from the water within the pitcher and the water in the pitcher loses heat and becomes cool. But in the rainy season, saturated air with water vapour slows down the rate of evaporation to a great extent. Thus, the water within the pitcher does not lose heat and hence it does not keep cool.
15) Why do the surroundings become pleasantly warm when freezing starts in cold countries?
Answer:
In cold countries, when freezing of water starts, it gives out its heat in the process of cooling up to 0 0 C. This heat makes the surrounding atmosphere less cold or pleasantly warmer.
16) Does land cool at a slower or faster rate than water? Give one reason for your answer.
Answer:
Land cools at a faster rate than water as the specific heat capacity of water (c = 4200 J kg -1 K -1 ) is about five times the specific heat capacity of land (800 J kg -1 K -1 ) and for a
given quantity of heat transfer Q and a given mass m, change in temperature ΔT is inversely proportional to the specific heat capacity.
i.e., (ΔT ∞ 1/c)
17) Why does a hot cup of tea cool down on adding sugar to it?
Answer:
When you add sugar to a hot beverage(tea) it is likely to dissolve faster. For dissolution of a compound into liquid, a transfer of heat takes place from liquid to the compound (here,
sugar). As the heat transfers to a hot substance to cold or room temperature substance, some heat of the tea gets transferred to the sugar, helping in its dissolution into it, thus making it somewhat cooler. Therefore a hot cup of tea cools down on adding sugar to it because sugar takes the heat (latent heat of fusion) from the tea and changes its state from solid to liquid.

18) Why does evaporation always produce cooling?
Answer:
Evaporation causes cooling – This is due to the fact that all molecules in a liquid do not have the same energy. Evaporation always produces cooling effect because some heat is required to evaporate the liquid. The particles of the liquid absorb energy from the surroundings to regain the energy lost during evaporation. This absorption of energy from the surroundings make the surroundings cold.

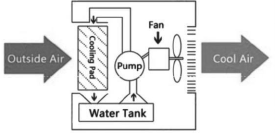
19) How does a desert cooler lower the temperature? Why is it not effective after the monsoon sets in?
Answer:
Desert cooler used in hot dry climate, works on the cooling produced by evaporation. A pump forces water to trickle through filters fitted on the three sides of a cubic metal box. A fan draws air from outside through the filters (water-soaked) and sends into the room. Forced evaporation of water produces cooling. But as the rain starts, it becomes humid and saturated with and hence, cooling is not produced.
20) Why does the heat supplied to a substance during its change of state not cause any rise in its temperature?
Answer:
When a substance changes from one state, or phase, of matter to another we say that it has undergone a change of state, or we say that it has undergone a change of phase.
Heat supplied to a substance during its change of state increases the potential energy of its molecules but no increase in kinetic energy. Since the rise in temperature is dependent on the kinetic energy of the molecules. Hence, no rise in its temperature takes place during its change of state.



