Exercise – 7 (A)
Question 1: Name the three classes in which elements are classified. Which was the first metal used by man?
Solution 1:
Metals, non-metals, and metalloids are the three classifications in which elements are categorized. The first metal that utilized by humans was copper.
Question 2: Name the metal which is a constituent of:
(a) Blood pigment, (b) Plant pigment
Solution 2:
(a) Iron (Fe) is the metal that is a contributor of blood pigment.
(b) Magnesium (Mg) is the metal that is a component of plant pigments.
Question 3: Give the importance of the following in living beings:
(a) Nitrogen (b) Hydrogen (c) Carbon
Solution 3:
(a) Nitrogen: Nitrogen is essential for production of amino acids, proteins, nucleic acid, etc. It is also used to preserve food.
(b) Hydrogen: It’s used to synthesize ghee by hydrogenating vegetable oils.
(c) Carbon: For growth and development in living beings, carbon plays a very crucial role.
Question 4: Name the metal and non-metal present in abundance in the earth crust.
Solution 4:
Aluminium is a metal that is abundant in the earth’s crust. Oxygen is a non-metal that is abundant in the crust of earth.
Question 5: Define metal and non-metal on the basis of electron loss or gain.
Solution 5:
Metals are defined as elements that lose electrons to produce positive ions. Non-metals are elements that acquire electrons and generate negative ions.
Question 6: State the position of the following in the periodic table:
(a) Alkali metals, (b) Alkaline earth metals
(c) Iron and zinc (d) Aluminium
Solution 6:
(a) Alkali metals: They belong to the IA group, which is the first column on the periodic table to the left.
(b)Alkaline earth metal: They belong to the IIA group, which is the second column on the periodic table to the left.
(c) Iron and Zinc: Cu is in the IB group, while Fe is in the VIII group.
(d) Aluminium: It belongs to the IIIA group, which is located on the right side of the periodic table.
Question 7: Give the general characteristics of:
Alkali metals,
Alkaline earth metals with reference to
(i) bonding (ii) action of air
(iii) action of water (iv) action of acid
Solution 7:
(i) Alkali metals with reference to:
Bonding: Ionic nature runs across all alkali metal salts.
Action of air: When they come into contact with oxygen and water vapour in the air, they react quickly.
Action of water: They Hydrogen gas is produced after a strong reaction with water.
2M + 2H2O ⟶ 2MOH + H2
Action of acid: They create hydrogen gas when they react aggressively with dilute HCl and dilute H2SO4.
2M + 2HCl ⟶ 2MCl + H2
(ii) Alkaline earth metal with reference to:
Bonding: Except for beryllium, all alkaline earth metal salts are ionic compounds.
Action of air: In comparison to alkali metals, they are less reactive.
Action of water: They create hydrogen gas when they react with water.
M + 2H2O ⟶ M(OH)2 + H2
Action of acid: They generate hydrogen gas whenever they react with dilute HCl and dilute H2SO4.
M + 2HCl ⟶ MCl2 + H2
Question 8: What are metalloids. Give examples.
Solution 8:
Metalloids are elements that have attributes of both metals and non-metals. Silicon and Germanium are some of the example of metalloids.
Question 9: Why is hydrogen placed with alkali metals?
Solution 9:
Hydrogen is placed with alkali metals as it has one electron in its valence shell similar to the alkali metals. That is why when hydrogen and alkali metals share one electron, they are classified altogether.
Question 10: Name:
(a) a liquid non-metal,
(b) a metal with dull appearance
(c) a metal with low melting and boiling points
(d) a non-metal with high m.p & b.p
(e) a metal which can float on water
(f) a metal which can be cut with a knife.
(g) a metal which is a bad conductor of heat and electricity
(h) a non-metal which is ductile
(i) a non- metal used in alloys
(j) a non-malleable metal
Solution 10:
(a) Bromine is a liquid non-metal.
(b) Lead is a metal with dull appearance.
(c) Gallium is a metal with low melting and boiling points.
(d) Carbon is a non-metal with high melting point and boiling point.
(e) Sodium is a metal which can float on water.
(f) Sodium is a metal which can be cut with a knife.
(g) Tungsten is a metal which is a bad conductor of heat and electricity.
(h) Carbon fibre is a non-metal which is ductile.
(i) Carbon is a non- metal used in alloys.
(j) Mercury is a non-malleable metal.
Question 11: Distinguish between metals and non metals on the basis of:
(i) ion formation, (ii) discharge of ions, (iii) nature of oxide formed, (iv) oxidizing and reducing property, (v) reaction with acids.
Solution 11:
(i) Ion formation: Ion formation is done when metals lose electrons to generate positive ions, whereas non-metals gain electrons to form negative ions.
(ii) Discharge of ions: During the process of electrolysis, metals are discharged from the cathode, whereas non-metals are freed from the anode.
(iii) Nature of oxide formed: Metal oxides are usually basic. Basic oxides dissolve in water to generate an alkaline solution, whereas non-metal oxides are typically acidic. Acidic solutions are formed when soluble acidic oxides dissolve in water.
(iv) Oxidizing and reducing property: Non-metals ionise by gaining electrons, making them oxidising agents, whereas metals ionise by losing electrons, making them reducing agents.
(v) Reaction with acids: Non-metals do not react with dilute hydrochloric or sulphuric acid, whereas metals above hydrogen in the activity series generally replace hydrogen from dilute non-oxidising acids.
Question 12: (a) Na – → Na+
(b) N + → N3-
(c) Cl + e– →
(d) Mg– → Mg2+
(e) M + HCl → MCl2+
(f) Mg + H2SO4 → ____ + ____
Solution 12:
(a) Na– e- → Na+
(b) N + 3e- → N3-
(c) Cl + e- → Cl-
(d) Mg– 2e → Mg2+
(e) M + 2HCl → MCl2 + H2
(f) Mg + H2SO4 → MgSO4 + H2
Question 13: Select from the following list:
Fe2O3, NO, PbO, Mn2O7
(a) Basic oxide………..
(b) Amphoteric oxide …………
(c) Acidic oxide ………………
(d) Neutral oxide …
Solution 13:
(a) Fe2O3 is basic oxide.
(b) PbO is amphoteric oxide.
(c) Mn2O7 is acidic oxide.
(d) NO is neutral oxide.
Question 14: Take an element from an alkali metal and one from an alkaline earth metal and write an equation for their action with:
(a) Hydrochloric acid (b) Oxygen (c) Sulphuric acid (d) Water.
Solution 14:
(a) Hydrochloric acid:
2Na + 2HCl → NaCl + H2
Be + 2HCl → BeCl2 + H2
(b) Oxygen:
4Na +O2 → 2Na2O
Mg + O2 → 2MgO
(c) Sulphuric acid:
2Na + H2SO4 → Na2SO4 + H2
Mg + H2SO4 → MgSO4 + H2
(d) Water:
2Na + H2O → 2NaOH + H2
Mg +H2O → Mg(OH)2 + H2
Exercise – 7 (B)
Question 1: Name:
(A) Two Metals Which Are Liquid At Room Temperature
(B) Two Metals Which Are Soft
(C) A Metal Which Lacks Ductility
(D) A Non Metal Which Is Lustrous
(E) A Non Metal Which Conducts Electricity
(F) A Metal Which Is Brittle
(G) Two Non Metals Which Are Monoatomic
(H) Two Metallic Oxides Which Are Acidic
(I) Two Metallic Oxides Which Are Amphoteric
(J) Two Metals Which React With Cold Water,
(K) The Compound Responsible For Green Deposit On The Surface Of Copper
(L) The Most Abundant Metal And The Most Abundant Non=Metal
(M) A Non Metal Which Can Form A Positive Ion
(N) A Non-Metal Which Shows Reducing Property
(O) A Metal Whose Oxide Is Reduced Only By Carbon
Solution 1:
(a) Mercury and gallium are two metals which are liquid at room temperature.
(b) Sodium and potassium are two metals which are soft.
(c) Mercury is a metal which lacks ductility.
(d) Iodine is a non metal which is lustrous.
(e) Graphite is a non metal which conducts electricity.
(f) Zinc is a metal which is brittle.
(g) Neon and argon are two non metals which are monoatomic.
(h) CrO3 and Mn2O7 are two metallic oxides which are acidic.
(i) Al2O3 and PbO are two metallic oxides which are amphoteric.
(j) Potassium and sodium are two metals which react with cold water.
(k) Basic copper(II) sulphate is the compound responsible for green deposit on the surface of copper.
(l) Aluminium and oxygen are the most abundant metal and the most abundant non-metal.
(m) Hydrogen is a non metal which can form a positive ion.
(n) Carbon is a non-metal which shows reducing property.
(o) Iron is a metal whose oxide is reduced only by carbon.
Question 2: Explain how the activity series accounts for each of the following:
(a) occurrence of metals (b) tendency to corrosion
(c) reaction with water (d) reaction with acids
Solution 2:
(a) Occurrence of metals: Metals at the top of the activity series are the most reactive, thus they are always found in the combined state, whereas metals at the bottom of the activity series are the least reactive, so they can also be found in the isolated state.
(b) Tendency to corrosion: Metals that are higher in the activity series than hydrogen may quickly react with moisture and air and corrode, but metals like gold and platinum cannot.
(c) Reaction with water: Moving down the line, the metals’ capacity to convert water to hydrogen diminishes.
Potassium and sodium are reactive with cold water whereas magnesium is reactive with warm water and aluminium, zinc and iron are reactive with steam.
(d) Reaction with acids: In the activity series, all metals above hydrogen convert hydrogen ions from diluted hydrochloric or sulphuric acid to produce hydrogen gas. As you progress through the series, the rate of reaction slows.
Question 3: Give the balanced reactions for the following:
(a) Sodium is dropped in water
(b) Magnesium reacts with boiling water
(c) Red hot iron reacts with steam
(d) Iron reacts with dilute HCI
Solution 3: (a) 2Na + 2H2O → 2NaOH + H2
(b) Mg + H2O → MgO + H2
(c) 3Fe +4H2O → Fe3O4 + 4H2
(d) Fe + 2HCl → FeCl2 + H2
Question 4: Give a short account of heating effect on metal carbonates based on the activity series.
Solution 4:
Higher in the activity series metals (such as Na and K) are stable at high temperatures and dissolved in water.
Metals such as Ca, Mg, Al, Zn, Fe, Pb, and Cu, on the other hand, disintegrate gradually to generate metal oxide and carbon dioxide when heated.
Metals lower in the activity series (e.g., Hg, Ag) breakdown to metal, oxygen, and carbon dioxide when heated.
Question 5: (a) Why are alkali metals kept in kerosene oil?
(b) What is:
basic lead carbonate and
brown powder deposit on iron?
(c) Why is hydrogen kept in the metal activity series?
Solution 5:
(a) Alkali metals like sodium and potassium react with moisture and air, that is why they are preserved in kerosene oil.
(b)
(i) Basic lead carbonate is a mixture of lead hydroxide and lead carbonate.
(ii) Brown powder is mainly hydrated iron (III) oxide (Fe2O3.xH2O)
(c) As Hydrogen can form a positive ion, hydrogen, while being a non-metal, has been included in the metal activity series. Based on the production of the positive ion, it would occupy the position.
Question 6: Give the effect of heat on metal oxides based on the activity series.
Solution 6:
Metal oxides such as Na, K, Ca, Mg, and Al are heat resistant and can only be reduced by electrolysis.
Coke is the sole way to decrease zinc oxide.
C, CO, H2, and NH3 decrease iron, lead, and copper oxides. Mercury and silver oxides breakdown into metal and oxygen.
Question 7: Metal A has an electronic configuration of 2, 8, 1 and metal B has 2, 8, 8, 2 which is more reactive metal.
(a) Identify A and B and give their reactions with dilute HCL and dilute H2SO4
(b) Give the effect of heat on their:
(i) oxides (ii) hydroxides (iii) carbonates (iv) nitrates
Solution 7:
Metal B is less reactive but Metal A is more reactive.
(a) Sodium (Na) is Metal A.
Calcium (Ca) is the metal B.
Reaction of Sodium (Na) with dilute HCl:
2Na + 2HCl → 2NaCl + H2
Ca + 2HCl → CaCl2 + H2
Reaction of Calcium (Ca) with dilute H2SO4 :
2Na + H2SO4 → Na2SO4 + H2
Ca + H2SO4 → CaSO4 + H2
(b)
(i) Oxides: Heat doesn’t affect sodium or calcium oxides and so can be reduced only by electrolysis.
(ii) Hydroxides: Calcium hydroxide decomposes into metal oxide and water vapour when heated, but sodium hydroxide remains stable.
(iii) Carbonates: Calcium carbonates disintegrate into calcium oxide and carbon dioxide when heated, whereas sodium carbonate remains stable.
(iv) Nitrates: Heating sodium nitrate produces nitrite and oxygen, but heating calcium nitrate produces calcium oxide, nitrogen dioxide, and oxygen.
Question 8: (a) The table below compares some properties of metals and non-metals. Write down the missing statements (i) to (iv):
| Metals | Non-metals |
| (i) ………………….. (ii) Malleable (iii) Form positive ions (iv) ………………… | (i) Poor conductors of heat (ii) …………………… (iii) …………………… (iv) Form acidic oxides |
(b) How many valence electrons are present in:
(i) Metals (ii) Non-metals?
Solution 8:
| Metals | Non-metals |
| (i) Good conductors of heat (ii) Malleable (iii) Form positive ions (iv) Form basic oxides | (i) Poor conductors of heat (ii) Non-Malleable (iii) Forms negative ions (iv) Form acidic oxides |
(b) Valence electrons present in:
Metals have 1, 2 or 3 valence electrons.
Non-metals have 5, 6 or 7 valence electrons.
Question 9: What is corrosion? What are necessary conditions for corrosion?
Solution 9:
The gradual destruction of the pure metals by the action of air, moisture or the chemical such as acid on their surface, the metal is said to corrode and the phenomenon is known as corrosion.
Necessary conditions for corrosion are:
(i) Oxygen and moisture levels are present.
(ii) Metals Metals towards the top of the activity scale corrode faster.
Question 10: State under what conditions corrosion is faster
Solution 10:
Conditions for increase of corrosion are:
(i) Oxygen and moisture levels are present.
(ii) Rusting is accelerated by contaminants such as NO2 and CO2.
(iii) Metals Metals towards the top of the activity scale corrode faster.
(iv) Corrosion is accelerated by dissolved ions in water, which function as an electrolyte.
Question 11: Corrosion can be an advantage in some case. Explain.
Solution 11:
Metal corrosion is advantageous because it protects the metal beneath it from additional harm. For example, when metals like aluminium and zinc are exposed to air, their oxides produce coatings that are exceedingly sticky and impermeable in nature, acting as a protective layer. This coating shields the metal from further deterioration.
Question 12: What is rust? Give the equation for the formation of rust.
Solution 12:
Rusting is the progressive deterioration of iron in the presence of water by environmental oxygen.
Equation:
4Fe + 3O2 +2xH2O → 2Fe2O3.xH2O
Question 13: State two conditions necessary for rusting of iron.
Solution 13:
The two conditions necessary for rusting of iron are:
(i) Air
(ii) Water
Question 14: How does the painting of an iron object prevent rusting?
Solution 14:
The iron does not come into touch with air reagents while it is painted. This keeps the metal from rusting.
Question 15: What is galvanization? How does it protect iron from rusting?
Solution 15:
Galvanisation is the process of treating steel or iron with a protective zinc coating to prevent corrosion.
The zinc coating prevents iron from rusting by preventing it from coming into touch with oxygen and moisture.
Question 16: A student has been collecting silver coins and copper coins. One day she observed a black coating on silver coins and a green coating on copper coins. Which chemical phenomenon is responsible for these coatings? Write the names of black and green coatings.
Solution 16:
When silver is exposed to air that contains the pollutant H2S, it tarnishes and creates a black coating of Ag2S.
When copper is exposed to damp air, it creates a green coating on its surface. This is generally copper (II) sulphate in its most basic form.
Question 17: Aluminium is said to be more reactive than iron, towards oxygen (or air) yet iron undergoes corrosion to a greater extent than aluminum. Explain.
Solution 17:
When aluminium is exposed to the air, it produces a white colour oxide. While iron interacts with air to generate a hydrated oxide called rust, this white colour oxide protects it from further corrosion. As a result, iron is more prone to corrosion.
Question 18: Which metals do not corrode easily?
Solution 18:
Gold and platinum, for example, are noble metals that do not corrode frequently.
Question 19: Why do gold ornaments look new even after several years of use?
Solution 19:
Gold is the least reactive metal, meaning it is unaffected by air, water, or other gases in the environment. As a result, gold is unaffected by corrosion. That’s why, even after years of usage, gold still looks sharp.
Exercise- 7 (C)
Question 1: Define the term ‘metallurgy’. State the processes involved in metallurgy.
Solution 1:
Metallurgy is the term describing the process of extracting metals in their purest form from their ores. It includes major steps i.e., concentration of the ore, the isolation of the metal from its concentrated ore and purification of metals.
The processes which are involved in metallurgy are stated below:
(i) Crushing and Grinding
(ii) Concentration
(iii) Roasting and Calcination
(iv) Reduction
(v) Refining
Question 2: Which metal occurs as:
(a) a sulphide (b) a halide (c) a carbonate (d) an oxide
Also give the names of their respective ores
Solution 2:
(a) Lead is a metal that occurs as a sulphide.
(b) Silver is a metal that occurs as a halide.
(c) Zinc is a metal that exists as a carbonate.
(d) Iron is a metal that occurs as an oxide.
Question 3: Distinguish between:
(a) a mineral and an ore,
(b) an ore and a metallic compound
Solution 3:
(a) Minerals are metal compounds that exist naturally in the presence of other substances such as soil, sand, limestone, and rocks. Ores are minerals from which metals may be economically mined at a reasonable cost and in a comfortable manner. Although it is not necessary that all ores are minerals, not all minerals are ores.
(b) Ores are minerals from which metals may be economically mined at a low cost and with little effort. A metallic compound is one in which one or more metal components are present. Examples: Silver nitrate (AgNO3) is a metallic chemical.
Question 4: Which metal can be extracted from each one of the following ores.
(a) bauxite (b) calamine (c) haematite
Solution 4:
The metals that can be extracted from the following ores are:
(a) Aluminium can be extracted from Bauxite.
(b) Zinc can be extracted from Calamine.
(c) Iron can be extracted from Haematite.
Question 5: State three objectives achieved during the roasting of ores.
Solution 5:
Three objectives achieved during the roasting of ores are as follows:
(a) It is used to extract moisture from ores.
(b) Sulphide ores are converted to oxides by this process.
(c) It increases the absorbency and reactivity of the ore.
(d) It eliminates contaminants that are volatile.
Question 6: Give the principles of:
(a) Hydrolytic method,
(b) Froth floatation
(c) Electromagnetic separation
Solution 6:
(a) Hydraulic washing: The key requirement for hydraulic washing is the density difference between the ore and the gangue.
(b) Forth floatation: This technique is based on the ore’s preferred water content with oil and the gangue particles’ preferential water sorption with water.
(c) Electromagnetic separation: Magnetic attributes of the ores are used in electromagnetic separation.
Question 7: Name:
the processes involved in
(a) concentration
(b) refining of ores
(c) two metallic oxides which cannot be reduced by carbon, carbon monoxide or hydrogen
Solution 7:
(a) The processes involved in concentration are:
Hydrolytic method
Magnetic Separation
Froth floatation
Leaching
(b) The processes involved in Refining of ores are:
Distillation
Liquation
Oxidation
Electro- refining
(c) Carbon, carbon monoxide, and hydrogen have little effect on potassium and sodium oxides.
Question 8: Explain the following terms:
(a) flux (b) gangue (c) slag (d) smelting
Solution 8:
(a) Flux: In a furnace, a flux is a substance that is injected to the charge to eliminate the gangue.
(b) Gangue: Gangue is a term for terrestrial contaminants like silica, mud, and other materials found in ore. Gangue is defined as unwanted material or impurities.
(c) Slag: When flux combines with impurities during metal recovery, it produces a fusible product.
(d) Smelting: Smelting is the process of reducing roasted oxide ore and removing gangue using a proper flux that is applied to the ore. It is a process of applying heat to ore in order to extract a base metal.
Question 9: Why does iron or zinc not occur free in nature?
Solution 9:
Iron and zinc are very reactive, they do not exist in their natural condition. Metals’ oxides, carbonates, and sulphides are natural metal compounds.
Question 10: What do you observe when hydrogen is passed over heated copper oxide?
Solution 10:
Copper oxide is converted from black to brown/red.

Question 11: Compare roasting and calcinations.
Solution 11:
Comparison of roasting and calcinations:

Question 12: (a) Name an ore of zinc.
(b) Which process is applied to concentrate it?
(c) How is concentrate d ore changes to oxide?
Solution 12:
(a) Most common zinc ore is sphalerite (zinc blende).
(b) Froth floatation is used to concentrate it.
(c) By heating ZnS in excess of air, concentrated ore is converted to oxide.
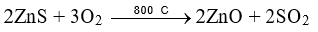
Question 13: Some metallic oxides can be reduced by hydrogen, carbon and carbon monoxide and some cannot. Explain.
Solution 13:
Because the oxides of highly active metals like potassium, sodium, calcium, magnesium, and aluminium have a strong affinity for oxygen, they cannot be reduced by carbon, carbon monoxide, or hydrogen.
The middle activity series metals (iron, zinc, lead, and copper) are highly reactive and do not exist in oxide form. In nature, they are found as sulphides or carbonates. These are first transformed to oxides, and then C, CO, or H2 can be used to reduce them.

Metals with low activity are less reactive, and their oxides can be converted to metals simply by heating.
Question 14: How are the following metallic oxides reduced. Write equations:
(a) Iron (II) oxide,
(b) Zinc oxide
Solution 14:
(a) Iron (II) oxide is reduced with the help of oxygen

Question 15: State why aluminium is extracted from its oxide by electrolysis while copper, lead, iron by reducing agents and mercury and silver by thermal decomposition.
Solution 15:
Aluminium is extracted from its ore due to its strong affinity for oxygen, aluminium cannot be reduced by carbon or carbon monoxide. Electrolysis is used to separate it from its oxide.
Metals like copper, lead, and iron are in the centre of the activity series and are moderately reactive, allowing carbon, CO, and hydrogen to decrease their oxides.
Mercury and silver are less reactive and fall further down the reactivity scale. By heating their oxides, these metals’ oxides are converted to metals.
Question 16: An ore on being heated in air forms sulphurous anhydride. Write the process used for the concentration of this ore.
Solution 16:
An ore on being heated in air forms sulphurous anhydride. Fractional distillation is the mechanism used to concentrate the ore.
Question 17: (a) On which factors does purification of metals depend?
(b) Name the methods used for purification.
(c) How is electro-refining done?
Solution 17:
(a) The purification is determined by:
Nature of the metal.
The nature of the metal’s impurities.
What will the metal be used for?
(b) Purification methods include:
(i) Oxidation
(ii) Distillation
(iii) Liquation
(iv) Electro – refining
(c) The anode is constructed of impure metal, whereas the cathode is formed of a thin sheet of pure metal. A salt solution of a metal to be refined is employed as an electrolyte. Impurities drop down and create anode mud as pure metal deposits at the cathode.


Solution 18:
Balanced equations are:

Exercise- 7 (D)
Question 1: State the position of aluminium in the periodic table.
Solution 1:
Position of Aluminium in the Periodic Table is herein under:
It holds 3rd position in Period,
It holds IIIA (13) in Group.
Question 2: Give the chemical names and formulae of any three ores of aluminium.
Solution 2:
The chemical names and formulae of the ores of aluminium are:
| Ore | Chemical name | Formula |
| Bauxite | Hydrated aluminium oxide | Al2O3.2H2O |
| Cryolite | Sodium aluminium | Na3AlF6 |
| Corundum | Fluoride Anhydrous aluminium oxide | Al2O3 |
Question 3: Which impurities are present in bauxite.
Solution 3:
The aluminium oxide content of bauxite ore is around 60%. Sand, ferric oxide, and titanium oxide make up the rest.
Question 4: What is red mud, how is it removed?
Solution 4:
Red mud is made up of ferric oxide, sand, and other materials that remain after bauxite dissolves in NaOH to generate sodium aluminate and is filtered out.
Red mud is termed as bauxite residue, is an industrial waste composed of various oxide compounds.
Question 5: Why electrolytic reduction is done to obtain aluminium?
Solution 5:
Aluminium is a stable compound because it has a high affinity for oxygen. It is resistant to typical reducing agents such as carbon, carbon monoxide, and hydrogen. As a result, electrolytic reduction is the method of choice for reducing alumina.
Question 6: Give the ionization reactions of electrolyte used in Hall’s process. Write the reaction at the cathode and the anode. Why the anode has to be replaced in this process?
Solution 6: In Hall’s approach, the electrolyte is ionised.
Cryolite:

The anode must be replaced on a regular basis because it is oxidised by the oxygen produced at the anode.
Question 7: Name the process by which the refining of aluminium is done.
Where are the cathode and anode in the electrolytic cell? Name the material used for these?
state the reactions at the two electrodes.
Solution 7:
(a) The electrolytic method used to refine aluminium is known as Hoope’s electrolytic process.
(b) The lowest layer is made of molten impure aluminium. The bottom layer is lined with carbon and serves as an anode.
(c) The cathode in the top layer is pure molten aluminium with carbon electrodes.
Reactions at the two electrodes are:
Anode: Al –3e– → Al3+
Cathode : Al3+ +3e– → Al
Question 8: How does aluminum react with the following:
(a) Air (b) Water (c) Acid (d)Base
Answer 8:
(a) Reaction of aluminum with Air:
At normal temperature, aluminum develops oxide.
With a strong light, aluminum powder burns at around 8000C in the air, creating oxide and nitride.
4Al + 3O2 → 2Al2O3
2Al + N2 → 2AlN
(b) Reaction of aluminum with Water:
Due to the sheer oxide layer on aluminum, water has no effect on it. Hydrogen is created when steam is passed over pure heated aluminum. 2Al + 3H2O ⟶ Al2O3 +3H2
(c) Reaction of aluminum with Water:
It produces salt and hydrogen when it combines with acids.
2Al + 6HCl → 2 AlCl3 +3H2
When dilute sulphuric acid combines with metal, hydrogen is produced.
2Al + 3H2SO4 (dilute) → Al2(SO4)3 +3H2
Sulphur dioxide is produced when concentrated sulphuric acid combines with aluminium.
2Al + 6H2SO4 → Al2(SO4)3 +6H2O +3SO2
Nitric acid, whether dilute and concentrated, has no effect on the metal aluminium.
(d) Reaction of aluminum with Base:
Aluminium produces meta aluminate when it interacts with boiling and dilute alkalies, whereas it produces aluminate when it reacts with fused alkalies.
2Al+ 2NaOH +2H2O → 2NaAlO2 + 3H2
(Sodium meta aluminate)
2Al + 6NaOH → 2Na3AlO3 + 3H2
(Sodium aluminate)
Question 9: What is the role of cryolite (Na3AIF6) in the electrolytic reduction of alumina in Hall’s process?
Solution 9:
The following are the functions of cryolite in Hall’s electrolytic reduction of alumina: 1. Lowers the fusion temperature from 20500C to 9500C and improves conductivity.
2. Improves conductivity, as pure alumina is a near-non-conductor of electricity.
3. The electrolytic combination is dissolved in cryolite, which functions as a solvent.
Question 10: Aluminium is a more active metal than iron, but suffers less corrosion. Why?
Explain and give reasons why aluminium vessels should not be cleaned with powders containing alkalis.
Solution 10:
Although aluminium is a more active metal, it oxidises and creates a thin protective coating on its surface that protects it from further corrosion.
It is not recommended to clean aluminium vessels using alkali-based powders since meta aluminates and hydrogen are formed.
2Al + 2NaOH + 2H2O ⟶ 2NaAlO2 + 3H2
Question 11: (a) Give the name and formula of the main orea of iron and zinc.
(b) How is the main ore of aluminium concentrated?
(c) Why ‘the food containing iron salts’ should not be cooked in aluminium utensils?
Solution 11:
(a) The main ores of iron and zinc are:
| Name | Ore | Formula |
| Iron Zinc | Red Haematite Zinc blende | Fe2O3 ZnS |

Question 12: Explain with reasons:
(a) In the electrolytic reduction of alumina, the graphite anode is gradually consumed.
(b) Roasting is carried out on sulphide ores and not on carbonate ores.
(c) Carbon can reduce lead oxide but not aluminium oxide
Solution 12:
(a) The graphite (anode) is consumed and must be replaced periodically during the electrolytic reduction of alumina because it is oxidised by oxygen to CO and further generates CO2.
2C + O2 ⟶ 2CO
2CO + O2 ⟶ 2CO2
(b) When metal sulphides are heated in excess of air, oxygen is required to convert them to metallic oxide and SO2. Carbonate is changed to oxide by CO2 loss, which occurs in the absence of air and when heated to a high temperature.
(c) Since aluminium has a strong affinity for oxygen, it cannot be reduced by carbon, carbon monoxide, or hydrogen, but lead oxide is easily reduced to metal lead by carbon.
PbO + C ⟶ Pb + CO
Question 13: (a) Why is flux used in the blast furnace?
(b) What does it form with silica present in the ore?
(c) How is it removed?
Solution 13:
(a) Flux reacts with gangue to generate slag, a flammable substance.
(b) It reacts with silica to generate slag[CaSiO3].
(c) It is taken from the higher exit, as slag is lighter than molten iron and floats on it.
Question 14: Name an ore which is concentrated by:
(a) forth floatation process,
(b) magnetic separation
Solution 14:
(a) Zinc blende [ZnS] is an ore which is concentrated by Froth flotation process.
(b) Haematite [Fe2O3] is an ore which is concentrated by Magnetic Separation.
Question 15: Distinguish between electrolytic methods of reduction and refining.
Solution 15:
Electrolytic Reduction
(i) It is the process of removing oxide or halide from a metal.
(ii) Electrolytic reduction of fused salts of highly active metals like Na, K, Ca, Mg, and Al reduces their oxides.
(iii) These metals’ oxides have a far stronger affinity for oxygen than carbon, and they cannot be reduced by carbon, CO, or hydrogen.
Electrolytic refining
Electrolytic refining of metals is the separation of residual impurities like Si and phosphorus.
(i) Presence of other metals and non-metals like Si and phosphorus.
(ii) Unreduced oxides and sulphides of metals.
It depends upon:
(i) It is dependent on the metal’s nature.
(ii) The metal is to be acquired for a specific purpose.
(iii) The type of contaminants those are present.
The anode is impure metal, whereas the cathode is a thin sheet of pure metal, and the electrolyte is a salt of a refined metal’s solution.
Question 16: Give three ways in which the metal zinc differs from the non-metals carbon. At least one of the differences must be a chemical difference.
Solution 16:
Metal zinc varies from non-metal carbon in three ways:
(a) Zinc has a valency of 2, whereas carbon has a valency of 4.
(b) Carbon, but not zinc, forms hydride (CH4).
(c) Zinc oxides are amphoteric (ZnO), whereas carbon oxides are acidic (CO2) and neutral (CO).
Exercise- 7 (E)
Question 1: State a reason why zinc is used in:
(a) galvanization (b) dry cells (c) cosmetics?
Solution 1:
(a) Zinc is used in the process of galvanization to prevent metal from getting corrode.
(b) Zinc is employed in dry cells because of its high electro positivity, which allows it to easily produce Zn+2 ions.
(c) Zinc is used as an antibacterial in face creams in cosmetics.
Question 2: State on what special properties the use of each of these metals depends:
(a) aluminium (b) zinc
Solution 2:
(a) Aluminium:
(i) It is utilised in alloys because it is a strong, light, and corrosion-resistant metal.
(ii) Aluminum is light, has a high tensile strength, is corrosion resistant, an excellent heat conductor, is unaffected by organic acids, and has a pleasing look. As a result, it’s utilised to make culinary utensils as well as in building and construction projects.
(iii) Since aluminium has a great affinity for oxygen, it is utilised as a deoxidizer in steel production.
(b) Zinc:
(i) Although zinc possesses a high electropositive property, it is used to coat iron and steel sheets to prevent corrosion, a process known as galvanization.
(ii) It is utilised to build dry cell containers that operate as negative electrodes because of its strong electropositive character, which produces Zn+2 ions.
(iii) Many organic reductions use zinc as a reducing agent, and these reductions are used to make pharmaceuticals and colours.
Question 3: Explain the following:
(a) Zinc is used to cover iron so as to prevent rusting of iron ehy?
(b) A neutral gas other than oxygen which is formed at the anode during electrolysis of fused alumina.
(c) Nitric acid can be stored in aluminium containers.
Solution 3:
(a) Zinc, being an electropositive metal, oxidises and preserves iron. Zinc also produces a protective coating of zinc oxide (ZnO) on iron. This coating is sticky and impermeable, and it prevents rusting on the iron metal beneath it.
(b) Carbon monoxide is a neutral gas other than oxygen that is produced at the anode during the electrolysis of fused alumina.
(c) Because nitric acid, both dilute and concentrated, does not react with metal, it may be stored in aluminium containers. Because an oxide coating forms on the surface of aluminium, it becomes passive.
Question 4: State the use of:
(a) cast iron (b) wrought Iron (c) Mild steel (d) hard steel.
Solution 4:
(a) Cast iron: Drain pipes, gutter covers, weights, and railings are all made of cast iron.
(b) Wrought iron: Chains, horseshoes, and electromagnets are all made of wrought iron.
(c) Mild steel: Mild steel is used to make nuts, bolts, and other fasteners.
(d) Hard Steel: Hard steel is a kind of steel that is used to produce tools.
Question 5: Which metal is used for:
(a) making pipes, buckets, water tanks,
(b) lithographic plates for printing
(c) making face creams
Solution 5:
(a) Galvanized iron sheets is used for making pipes, buckets, water tanks.
(b) Zinc is used for lithographic plates for printing.
(c) Zinc is used for making face creams.
Question 6: Give reasons, why aluminum is used in:
(a) making alloys
(b) wrapping chocolates
(c) painting electric and telegraphic poles
(d) In aluminiothermy
(e) In making ships
Solution 6:
(a) Aluminium is used to make alloys because it is a strong, light, and corrosion-resistant metal.
(b) Aluminium is used to wrap chocolates because it is light, pliable, and does not corrode.
(c) Electric and telegraphic poles are painted with aluminium to keep them from rusting.
(d) Since it is an excellent reducing agent, it is employed in aluminothermy.
(e) Aluminium protects ships against corrosion by forming an aluminium oxide coating. As a result, it’s employed in the construction of ships.
Question 7: Aluminum is used in thermite welding:
(a) What is thermit?
(b) What is ignition mixture?
(c) Write reaction for process?
Solution 7:
(a) A mixture of three parts of ferric oxide (Fe2O3) and one part of aluminium powder (Al).
(b) The ignition combination is made up of potassium chlorate and magnesium powder.
(c) Reaction: Fe2O3 + 2Al → Al2O3 + 2Fe + heat
Question 8: What is an alloy? How do the properties of an alloy differ from its constituents?
Solution 8:
A homogeneous mixture of two or more metals, or one or more metals with certain non-metallic components, is referred to as an alloy.
The characteristics of alloys are frequently dissimilar to those of their constituents. Consider the following scenario:
Without a little proportion of copper, gold is too soft to be utilised.
Steel with a low molybdenum content is tougher and more resistant to wear. Copper and tin are less sonorous than bell metal.
Alnico, an aluminium, nickel, and cobalt alloy, can lift 60 times its own weight.
Hardness, wear resistance, toughness, and other qualities are improved by the addition of these components.
Question 9: Name three alloys of steel. Give their compositions and uses.
Solution 9:
| Alloy’s name | Composition | Uses |
| 1. Stainless steel | 73% Fe, 18%Cr, 8%Ni, 1%C | Used for making utensils, cutlery, ornamental pieces and surgical instruments. |
| 2. Manganese steel | 85% Fe, 1%C , 14%Mn | Used for making rock drills |
| 3. Tungsten steel | 84%Fe, 5%W, 1%C | and armour plates. Used for cutting tools for high speed athes. |
Question 10: Both brass and bronze contain copper as major constituents. Name other elements in these alloys.
Solution 10:
(i) Zinc is the other component of Brass.
(ii) Tin and Zinc are the other components in Bronze.
Question 11: Name an alloy of:
(a) aluminium used in aircraft construction
(b) lead used in electrical wiring or electrical work in joining metals.
(c) copper in electrical appliances or household vessels
(d) zinc used in simple voltaic cells
Solution 11:
(a) Duralumin is an alloy of aluminium used in aircraft construction.
(b) Solder is an alloy of lead used in electrical wiring or electrical work in joining metals.
(c) Brass is an alloy of copper in electrical appliances or household vessels.
(d) Zinc amalgam is an alloy of zinc used in simple voltaic cells.
Question 12: What is an amalgam? State its use with an example.
Solution 12:
Amalgam is a combination or alloy of mercury with a variety of metals or alloys such as sodium, zinc, gold, and silver, as well as various non-metals.
Dental amalgam is a mercury-silver tin alloy combination.
Question 13: (a) State two properties of brass that render it more useful for some purpose than its components.
(b) A metal which forms a liquid alloys at ordinary temperature.
Solution 13:
(a) Brass has two characteristics that make it more useful than its constituents:
(i) It is malleable and ductile.
(ii) It is corrosion resistant.
(iii) Is simple to cast.
(b) Sodium is a metal that forms a liquid alloy at room temperature.
Question 14: What is magnalium? Name the main elements present in it? Write its one use.
Solution 14:
Magnalium is an aluminum-magnesium alloy having a 90-95 percent aluminium content and a 10-5 percent magnesium content. It’s utilised in the manufacture of aeroplanes.
Question 15: Name the constituents of:
(a) Duralumin (b) solder, (c) Bronze (d) Invar
Solution 15:
The constituents of:
(a) The constituents of Duralumin are aluminium (95%), copper (4%), magnesium (0.5%) and manganese (0.5%).
(b) The constituents of Solder are lead (50%) and tin (50%).
(c) The constituents of Bronze are copper (80%), tin (18%) and zinc (2%).
(d) The constituents of Invar are iron (63%), nickel (36%) and carbon (1%).
Miscellaneous Exercise
Question 1: For each substance listed below, explain its significance in the extraction of aluminium.
(a) bauxite (b) Sodium hydroxide (c) Cryolite (d) Graphite
Solution 1:
(a) Bauxite: Aluminium is derived from the bauxite mineral Al2O3.2H2O, which is its principal resource. It has a 60 percent Al2O3 concentration. (b) Sodium hydroxide: Sodium hydroxide dissolves bauxite to generate sodium meta aluminate and forms red mud to remove insoluble impurities from Al2O3

(c) Cryolite: Cryolite decreases the melting point from 20500 to 9500 degrees Celsius and improves conductivity.
(d) Graphite: Graphite serves as both a cathode and an anode. Its role is to prevent the formation of O2 at the anode otherwise aluminium may be oxidized by oxygen.
Question 2: From the metals: copper, iron, magnesium, sodium and zinc, select a different metal in each case which:
(a) does not react with dilute hydrochloric acid
(b) can form 2 + and 3 + ions
(c) has a hydroxide that reacts with both acids and alkalis
(d) for not react with cold water but reacts with steam when heated.
Solution 2:
(a) Copper does not react with dilute hydrochloric acid.
(b) Iron can form 2 + and 3 + ions.
(c) Zinc has a hydroxide that reacts with both acids and alkalis.
(d) Magnesium does not react with cold water but reacts with steam when heated.
Question 3: Arrange the metals in (2) in the decreasing order of reactivity.
Solution 3:
Metals are arranged in decreasing order of reactivity as follows:
Sodium > Magnesium > Zinc > Iron > copper
Question 4: In order to obtain 1 tonne of aluminium the following inputs are required. 4 tonnes of bauxite, 150 kg of sodium hydroxide and 600 kg of graphite. The aluminium compound in bauxite is
aluminium oxide and the main impurity is iron (III) oxide, Aluminium is obtained by the electrolysis of aluminium oxide dissolved in cryolite
(a) when bauxite is treated with sodium hydroxide solution, what happens to
i. the aluminium oxide
ii. the iron (III) oxide
(b) i. Name the process used for the purification of bauxite
ii. Write the equation for the action of heat on aluminium hydroxide
(c) i. write the formulae of cryolite
ii. Write down the word which correctly completes the following sentences. By dissolving aluminium oxide in cryolite a __________ (conducting/non conducting) solution is produced.
iii. why is so much graphic required for the electrolytic process?
iv. Write the equation for the reaction which takes place at cathode.
(d) In construction work, why is the alloy of alluminium duralumin used rather than pure aluminium ?
Solution 4:
(a)
i. Sodium aluminate is formed when aluminium oxide reacts with water.

ii. The iron(III) oxide is eliminated by filtering since it does not breakdown in NaOH.
(b)
i. The Bayer’s procedure is the name of the purifying method for bauxite.
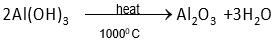
ii. Heat action on aluminium hydroxide is described by the following equation:
(c)
i. The cryolite’s formula is Na3AlF6.
ii. By dissolving aluminium oxide in cryolite a conducting solution is produced.
iii. The graphite functions as an anode in the electrolytic process. The anode must be replaced on a regular basis because it is oxidised by the oxygen produced at the anode.
The reaction that takes place at the cathode is:
4Al3+ +12e– → Al
(d) Duralumin, an aluminium alloy, is used in construction because it is durable and corrosion resistant.
Question 5: Aluminium is extracted from its chief ore, bauxite. The ore is first purified and then the metal is extracted from it by electrolytic reduction.
(a) Write three balanced equation for the purification of bauxite
(b) Name a chemical used for dissolving aluminium oxide, In which state of subdivision is the chemical used?
(c) Write an equation for the reaction which takes place at the anode during the extraction of aluminium by the electrolytic process.
(d) Mention one reason for the use of aluminium in thermite welding.
Solution 5:
(a) The following are three balanced equations for bauxite purification:

(b) Al2O3 is dissolved using fluorspar and cryolite. In the intermediate state, this chemical is utilised.
(c) Reaction at anode:
6O-2 – 12e– → 6[O]
3O + 3O → 3O2
The anode is converted to carbon monoxide, which is subsequently converted to carbon dioxide.
2C + O2 → 2CO
2CO +O2 → 2CO2
(d) The reduction of aluminium in thermite welding is very exothermic, and the heat generated is sufficient to melt the metal.
Fe2O3 + 2Al → 2Fe + Al2O3 + Heat
Question 1: (a) A to F below relate to the source and extraction of either zinc or aluminium:
A. Bauxite
B. Coke
C. Cryolite
D. Froth floatation
E. Sodium hydroxide solution
F. Zinc blende
i. Write down the three letters each from the above list which are relevant to:
1. Zinc 2. Aluminum
ii. Fill in the blanks using the most appropriate words from A to F.
1. The ore from which aluminum is extracted must first be treated with____________ so that pure aluminum oxide can be obtained.
Solution 1:
i. Froth Flotation, Zinc Blende and Coke are related to Zinc.
Bauxite, Cryolite and Sodium hydroxide solution are relevant to Aluminium.
ii. The ore from which aluminum is extracted must first be treated with Sodium hydroxide so that pure aluminum oxide can be obtained.
Question 2: Calcium copper, lead aluminium zinc chromium, magnesium and iron.
Choose the major metals from the list given above to make the following alloys:
(a) Stainless steel
(b) brass
Solution 2:
(a) Alloy stainless steel is made up of Iron and Chromium.
(b) Alloy brass is made up of Copper and Zinc.
Question : Name the following:
(a) A metal which is liquid at room temperature.
(b) The process of heating an ore to a high temperature in the presence of air.
(c) The compound formed by the reaction between calcium oxide and silica.
(d) A compound which is added to lower the fusion temperature of the electrolytic bath in the extraction of aluminium.
(e) Name an allotrope of a non-metal that allows electricity to pass through it.
Solution :
(a) Mercury
(b) Roasting
(c) CaSiO3
(d) Cryolite
(e) Graphite
Question 1: From the list of characteristics given below, select the five which are relevant to non-metals and their compounds:
A. Ductile
B. Conduct electicity
C. Brittle
D. Acidic oxide
E. Basic oxides
F. Discharge at anode
G. Discharge at cathode
H. Ionic chlorides
I. Covalent chlorides
J. Reaction with dilute sulphuric acid yields hydrogen,
K. 1, 2, or 3 valence electrons
L. 5, 6, 7 valence electrons
(write the five letters corresponding to the correct characteristics)
Solution 1:
The five characteristics which are relevant to non-metals and their compounds:
They are Brittle, Acidic oxide, Discharged at anode, Covalent chlorides, 5,6,7 valence electrons.
Question 2: The following is an extract from ‘Metals in the service of Man, Alexander and street/pelican 1976:
‘Alumina (aluminium oxide) has a very high melting point of over 2000° C so that it cannot readily be liquefies .However conversion of alumina to aluminium and oxygen, by electrolysis can occur when it is dissolved in some other substance’.
(i) Which solution is used to react with bauxide as a first step in obtaining pure aluminium oxide?
(ii) The aluminium oxide for the electrolytic extraction of aluminum is obtained by heating aluminium hydroxide. Write the equation for this reaction.
(iii) Name the element which serves both as the anode and the cathode in the extraction of aluminum.
(iv) Write the equation for the reaction that occurs at the cathode during the extraction of aluminium by electrolysis.
(v) Give the equation for the reaction which at the anode when aluminum is purifies by electrolysis.
Solution 2:
(i) Sodium hydroxide solution is used to react with bauxide as a first step in obtaining pure aluminium oxide.
(ii) The aluminium oxide for the electrolytic extraction of aluminum is obtained by heating aluminium hydroxide.

(iii) Graphite is the element which serves both as the anode and the cathode in the extraction of aluminum.
(iv) Reaction at cathode:
Al3+ + 3e- ¾¾® Al
(v) Reaction at anode:
Al – 3e– ¾¾® Al3+
Question 1: The following is a sketch of an electrolytic cell used in the extraction of aluminium:
(i) What is the substance of which the electrodes A and B are made?
(ii) At which electrode (A or B) is the aluminum formed?
(iii) What are the two aluminum compounds in the electrolyte C?
(iv) Why is it necessary for electrode B to be continuously replaced?
Solution 1:
(i) A is a carbon rod, while B is a thick graphite rod.
A⟶ Cathode
B ⟶ Anode
(ii) At electrode A, aluminium is produced.
(iii) In the electrolyte C, the two aluminium compounds are Na3AlF6 and Al2O3.
(iv) Since electrode B is oxidised by the oxygen released, it must be replaced on a regular basis.
Question 2: Brass is an alloy of:
Copper and tin,
Copper and Zic
Zinc and lead,
Lead and tin.
Solution 2:
Brass is an alloy of copper and Zinc.





